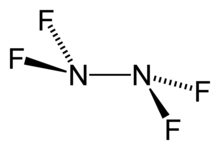
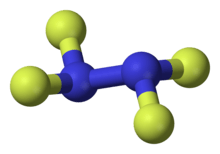
Tetrafluorohydrazine
Tetrafluorohydrazine or dinitrogen tetrafluoride, N2F4, is a colourless, reactive inorganic gas. It is a fluorinated analog of hydrazine. It is a highly hazardous chemical that explodes in the presence of organic materials.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2-tetrafluorohydrazine | |
| Other names
dinitrogen tetrafluoride, perfluorohydrazine, UN 1955 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.091 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N2F4 | |
| Molar mass | 104.01 g mol−1 |
| Melting point | −164.5 °C (−264.1 °F; 108.6 K) [1] |
| Boiling point | −73 °C (−99 °F; 200 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrafluorohydrazine is manufactured from nitrogen trifluoride using an iron catalyst or iron(II) fluoride. It is used in some chemical syntheses, as a precursor or a catalyst.
Tetrafluorohydrazine was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959.[2]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Tetrafluorohydrazine at DTIC.mil archived March 12, 2007
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.