Calcium fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.262 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CaF2 | |||
| Molar mass | 78.075 g·mol−1 | ||
| Appearance | White crystalline solid (single crystals are transparent) | ||
| Density | 3.18 g/cm3 | ||
| Melting point | 1,418 °C (2,584 °F; 1,691 K) | ||
| Boiling point | 2,533 °C (4,591 °F; 2,806 K) | ||
| 0.015 g/L (18 °C) 0.016 g/L (20 °C) | |||
Solubility product (Ksp) |
3.9 × 10−11 [1] | ||
| Solubility | insoluble in acetone slightly soluble in acid | ||
| -28.0·10−6 cm3/mol | |||
Refractive index (nD) |
1.4338 | ||
| Structure | |||
| cubic crystal system, cF12[2] | |||
| Fm3m, #225 | |||
| Ca, 8, cubic F, 4, tetrahedral | |||
| Hazards | |||
| Main hazards | Reacts with concentrated sulfuric acid to produce hydrofluoric acid | ||
| Safety data sheet | ICSC 1323 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published) |
>5000 mg/kg (oral, guinea pig) 4250 mg/kg (oral, rat)[3] | ||
| Related compounds | |||
Other anions |
Calcium chloride Calcium bromide Calcium iodide | ||
Other cations |
Beryllium fluoride Magnesium fluoride Strontium fluoride Barium fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chemical structure
The compound crystallizes in a cubic motif called the fluorite structure.
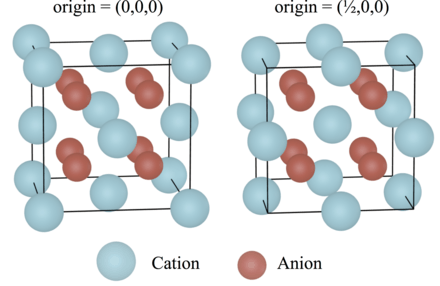
Ca2+ centres are eight-coordinate, being centered in a cube of eight F− centres. Each F− centre is coordinated to four Ca2+ centres in the shape of a tetrahedron.[5] Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers. The same crystal structure is found in numerous ionic compounds with formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding anti-structure, called the antifluorite structure, anions and cations are swapped, such as Be2C.
Preparation
The mineral fluorite is abundant, widespread, and mainly of interest as a precursor to HF. Thus, little motivation exists for the industrial production of CaF2. High purity CaF2 is produced by treating calcium carbonate with hydrofluoric acid:[6]
- CaCO3 + 2 HF → CaF2 + CO2 + H2O
Applications
Naturally occurring CaF2 is the principal source of hydrogen fluoride, a commodity chemical used to produce a wide range of materials. Calcium fluoride in the fluorite state is of significant commercial importance as a fluoride source.[7] Hydrogen fluoride is liberated from the mineral by the action of concentrated sulfuric acid:[8]
- CaF2 + H2SO4 → CaSO4(solid) + 2 HF
Niche uses
Calcium fluoride is used to manufacture optical components such as windows and lenses, used in thermal imaging systems, spectroscopy, telescopes, and excimer lasers. It is transparent over a broad range from ultraviolet (UV) to infrared (IR) frequencies. Its low refractive index reduces the need for anti-reflection coatings. Its insolubility in water is convenient as well. Doped calcium fluoride, like natural fluorite, exhibits thermoluminescence and is used in thermoluminescent dosimeters. It forms when fluorine combines with calcium.
Safety
CaF2 is classified as "not dangerous", although reacting it with sulfuric acid produces very toxic hydrofluoric acid. With regards to inhalation, the NIOSH-recommended concentration of fluorine-containing dusts is 2.5 mg/m3 in air.[6]
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- X-ray Diffraction Investigations of CaF2 at High Pressure, L. Gerward, J. S. Olsen, S. Steenstrup, M. Malinowski, S. Åsbrink and A. Waskowska, Journal of Applied Crystallography (1992), 25, 578-581 doi:10.1107/S0021889892004096
- "Fluorides (as F)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Burr, P. A.; Cooper, M. W. D. (2017-09-15). "Importance of elastic finite-size effects: Neutral defects in ionic compounds". Physical Review B. 96 (9): 094107. arXiv:1709.02037. Bibcode:2017PhRvB..96i4107B. doi:10.1103/PhysRevB.96.094107.
- G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_307. ISBN 3527306730.
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, Renée; Cuer, Jean Pierre (2005), "Fluorine Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, p. 307, doi:10.1002/14356007.a11_307.
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
External links
- NIST webbook thermochemistry data
- Charles Townes on the history of lasers
- National Pollutant Inventory - Fluoride and compounds fact sheet
- Crystran Material Data
- MSDS (University of Oxford)