Tellurium tetrafluoride
Tellurium tetrafluoride, TeF4, is a stable, white, hygroscopic crystalline solid and is one of two fluorides of tellurium. The other binary fluoride is tellurium hexafluoride.[1] The widely reported Te2F10 has been shown to be F5TeOTeF5 [1] There are other tellurium compounds that contain fluorine, but only the two mentioned contain solely tellurium and fluorine. Tellurium difluoride, TeF2, and ditellurium fluoride, Te2F are not known.[1]
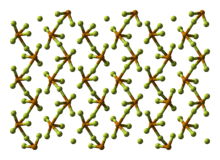 | |
| Names | |
|---|---|
| IUPAC name
tellurium(IV) fluoride | |
| Identifiers | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| TeF4 | |
| Molar mass | 203.594 |
| Appearance | white crystalline solid |
| Melting point | 129 °C (264 °F; 402 K) |
| Related compounds | |
Other anions |
tellurium dioxide, tellurium tetrachloride, tellurium(IV) bromide, tellurium(IV) iodide |
Other cations |
sulfur tetrafluoride, selenium tetrafluoride |
Related compounds |
tellurium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Tellurium tetrafluoride can be prepared by the following reaction:
It is also prepared by reacting nitryl fluoride with tellurium or from the elements at 0 °C or by reacting selenium tetrafluoride with tellurium dioxide at 80 °C.
Fluorine in nitrogen can react with TeCl2 or TeBr2 to form TeF4. PbF2 will also fluorinate tellurium to TeF4.
Reactivity
Tellurium tetrafluoride will react with water or silica and forms tellurium oxides. Copper, silver, gold or nickel will react with tellurium tetrafluoride at 185 °C. It does not react with platinum. It is soluble in SbF5 and will precipitate out the complex TeF4SbF5.
Properties

Tellurium tetrafluoride melts at 130 °C and decomposes to tellurium hexafluoride at 194 °C. In the solid phase it consists of infinite chains of TeF3F2/2 in an octahedral geometry. A lone pair of electrons occupies the sixth position.
References
- R.B. King; Inorganic Chemistry of Main Group Elements, VCH Publishers, New York, 1995.
- W.C. Cooper; Tellurium, VanNostrand Reinhold Company, New York, 1971.
- Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5