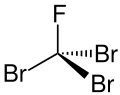
Tribromofluoromethane
Tribromofluoromethane or Halon 1103 or R 11B3 is a fully halogenated mixed halomethane or more exactly a bromofluorocarbon (BFC). It is a colorless liquid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tribromo(fluoro)methane | |
| Other names
Tribromofluoromethane Tribromo-fluoro-methane Fluorotribromomethane Halon 1103 FC-11B3 R 11B3 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.942 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CBr3F | |
| Molar mass | 270.72 g/mol |
| Appearance | Colorless liquid |
| Density | 2.7650 g/cm3 at 25 °C
1.8211 g/cm3 at 0 °C |
| Melting point | −73 °C (−99 °F; 200 K) |
| Boiling point | 108 °C (226 °F; 381 K) |
| Hazards | |
| Main hazards | Irritant (Xi) |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26, S37/39 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tribromofluoromethane can be used in fire extinguishers. However, because it contains bromine, it has high ozone depletion potential (ODP) and most of the halons are banned by Montreal Protocol.
Table of physical properties
| Property | Value |
|---|---|
| Refractive index (n) at 20 °C | 1.5216 |
| Surface tension at 20 °C | 31.68 mN.m−1 |
| Viscosity at 0 °C | 2.09 mPa.s (2.09 cP) |
gollark: They seem to mostly use replicators to conveniently make food?
gollark: Why not? AUTOPILOT™.
gollark: Again, replicators. They barely use them despite them being VERY USEFUL.
gollark: It mostly goes by "cool and sounds good", not "actually makes any sense".
gollark: Star Trek is not exactly hugely realistic.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.