Cyanogen
Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C−C≡N, although other isomers have been detected.[6] The name is also used for the CN radical,[7] and hence is used for compounds such as cyanogen bromide (NCBr).[8]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalonitrile[1] | |||
| Systematic IUPAC name
Ethanedinitrile[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1732464 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.643 | ||
| EC Number |
| ||
| 1090 | |||
| MeSH | cyanogen | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1026 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| (CN)2 | |||
| Molar mass | 52.034 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | pungent, almond-like | ||
| Density | 950 mg mL−1 (at −21 °C) | ||
| Melting point | −28 °C (−18 °F; 245 K) | ||
| Boiling point | −21.1 °C; −6.1 °F; 252.0 K | ||
| 45 g/100 mL (at 20 °C) | |||
| Solubility | soluble in ethanol, ethyl ether | ||
| Vapor pressure | 5.1 atm (21 °C)[5] | ||
Henry's law constant (kH) |
1.9 μmol Pa−1 kg−1 | ||
| -21.6·10−6 cm3/mol | |||
Refractive index (nD) |
1.327 (18 °C) | ||
| Thermochemistry | |||
Std molar entropy (S |
241.57 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
309.07 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.0978–−1.0942 MJ mol−1 | ||
| Hazards | |||
| Main hazards | forms cyanide in the body; flammable[5] | ||
| Safety data sheet | inchem.org | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H220, H331, H400, H410 | ||
| P210, P261, P271, P273, P304+340, P311, P321, P377, P381, P391, P403, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 6.6–32%[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[5] | ||
REL (Recommended) |
TWA 10 ppm (20 mg/m3)[5] | ||
IDLH (Immediate danger) |
N.D.[5] | ||
| Related compounds | |||
Related alkanenitriles |
|||
Related compounds |
DBNPA | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Cyanogen is the anhydride of oxamide:
- H2NC(O)C(O)NH2 → NCCN + 2 H2O
although oxamide is manufactured from cyanogen by hydrolysis:[9]
- NCCN + 2 H2O → H2NC(O)C(O)NH2
Preparation
Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide:
- 2 Hg(CN)2 → (CN)2 + Hg2(CN)2
Alternatively, one can combine solutions of copper(II) salts (such as copper(II) sulfate) with cyanides, an unstable copper(II) cyanide is formed which rapidly decomposes into copper(I) cyanide and cyanogen.[10]
- 2 CuSO4 + 4 KCN → (CN)2 + 2 CuCN + 2 K2SO4
Industrially, it is created by the oxidation of hydrogen cyanide, usually using chlorine over an activated silicon dioxide catalyst or nitrogen dioxide over a copper salt. It is also formed when nitrogen and acetylene are reacted by an electrical spark or discharge.[11]
Isomers
Cyanogen is NCCN. There are less stable isomers in which the order of the atoms differs. Isocyanogen (or cyanoisocyanogen) is NCNC, diisocyanogen is CNNC, and diazodicarbon(citation needed) is CCNN.
Paracyanogen
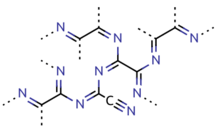
Paracyanogen is a polymer of cyanogen. It can be best prepared by heating mercuric cyanide. It can also be prepared by heating silver cyanide, silver cyanate, cyanogen iodide or cyanuric iodide.[12] It can also be prepared by the polymerization of cyanogen at 300 to 500 °C (572 to 932 °F) in the presence of trace impurities. Paracyanogen can also be converted back to cyanogen by heating to 800 °C (1,470 °F).[9] Based on experimental evidence, the structure of this polymeric material is thought to be rather irregular, with most of the carbon atoms being of sp2 type and localized domains of π conjugation.[13]
History
Cyanogen was first synthesized in 1815 by Joseph Louis Gay-Lussac, who determined its empirical formula and named it. Gay-Lussac coined the word "cyanogène" from the Greek words κυανός (kyanos, blue) and γεννάω (gennao, I create), because cyanide was first isolated by the Swedish chemist Carl Wilhelm Scheele from the pigment "Prussian blue".[14] By the 1850s, cyanogen soap was used by photographers to remove silver stains from their hands.[15] It attained importance with the growth of the fertilizer industry in the late 19th century and remains an important intermediate in the production of many fertilizers. It is also used as a stabilizer in the production of nitrocellulose.
In 1910 a spectroscopic analysis of Halley's Comet found cyanogen in the comet's tail, which led to public fear that the Earth would be poisoned as it passed through the tail. Because of the extremely diffuse nature of the tail, there was no effect when the planet passed through it.[16][17]
Safety
Like other cyanides, cyanogen is very toxic, as it readily undergoes reduction to cyanide, which poisons the cytochrome c oxidase complex, thus interrupting the mitochondrial electron transfer chain. Cyanogen gas is an irritant to the eyes and respiratory system. Inhalation can lead to headache, dizziness, rapid pulse, nausea, vomiting, loss of consciousness, convulsions, and death, depending on exposure.[18] Lethal dose through inhalation typically ranges from 100 to 150 milligrams (1.5 to 2.3 grains). Inhalation of 900 ppm over a period of 10 minutes is considered lethal.[19]
Cyanogen produces the second-hottest-known natural flame (after carbon subnitride) with a temperature of over 4,525 °C (8,177 °F) when it burns in oxygen.[20][21]
In popular media
In the 5th episode of season 13 Doctor Who The Brain of Morbius the doctor synthesizes cyanogen using hydrogen cyanide as a starting material and vents it through a pipe to stop Solon from performing surgery on the brain of Morbius’s body, however he completes it but shortly after dies to cyanogen poisoning.
In Dragnet (1987) Friday (Dan Ackroyd) and Streebek (Tom Hanks) were tracking down the villain who stole "the pseudohallogenic compound Cyanagen". http://www.subzin.com/quotes/M100518da4/Dragnet/The+trichlornitromethane+and+the+pseudo-halogenic+compound+cyanogen.
See also
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 902. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- "oxalonitrile (CHEBI:29308)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 27 October 2006. Main. Retrieved 6 June 2012.
- NIOSH Pocket Guide to Chemical Hazards. Department of Health and Human Services, Centers for Disease Control, National Institute for Occupational Safety & Health. September 2007. p. 82.
- The Merck Index (10th ed.). Rahway, NJ: Merck & Co. 1983. p. 385.
- NIOSH Pocket Guide to Chemical Hazards. "#0161". National Institute for Occupational Safety and Health (NIOSH).
- Ringer, A. L.; Sherrill, C. D.; King, R. A.; Crawford, T. D. (2008). "Low-lying singlet excited states of isocyanogen". International Journal of Quantum Chemistry. 106 (6): 1137–1140. Bibcode:2008IJQC..108.1137R. doi:10.1002/qua.21586.
- Irvine, William M. (2011). "Cyanogen Radical". Encyclopedia of Astrobiology. p. 402. doi:10.1007/978-3-642-11274-4_1806. ISBN 978-3-642-11271-3.
- Hartman, W. W.; Dreger, E. E. (1931). "Cyanogen Bromide" (PDF). Organic Syntheses. 11: 30.; Collective Volume, 2, p. 150
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 320–321. ISBN 978-0-08-037941-8.
- Brotherton, T. K.; Lynn, J. W. (1959). "The Synthesis And Chemistry Of Cyanogen". Chemical Reviews. 59 (5): 841–883. doi:10.1021/cr50029a003.
- Breneman, A. A. (January 1889). "The Fixation of Atmospheric Nitrogen". Journal of the American Chemical Society. 11 (1): 2–27. doi:10.1021/ja02126a001.
- Bircumshaw, L. L.; F. M. Tayler; D. H. Whiffen (1954). "Paracyanogen: its formation and properties. Part I". J. Chem. Soc.: 931–935. doi:10.1039/JR9540000931.
- Maya, Leon (1993). "Paracyanogen Reexamined". Journal of Polymer Science Part A (Submitted manuscript). 31 (10): 2595–2600. Bibcode:1993JPoSA..31.2595M. doi:10.1002/pola.1993.080311020.
- Gay-Lussac, J. L. (1815). "Recherches sur l'acide prussique". Annales de Chimie. 95: 136–231. Gay-Lussac names cyanogen on p. 163.
- Crookes, William, ed. (1859). "Photographic News: A Weekly Record of the Process of the Photography": 11. Cite journal requires
|journal=(help) - Comet's Poisonous Tail.
- Halley's Comet 100 years ago.
- Muir, G. D., ed. (1971). Hazards in the Chemical Laboratory. London: The Royal Institute of Chemistry.
- Ledgard, Jared (2006). A Laboratory History of Chemical Warfare Agents. Lulu.com. ISBN 978-0615136455. p. 82.
- Thomas, N.; Gaydon, A. G.; Brewer, L. (1952). "Cyanogen Flames and the Dissociation Energy of N2". The Journal of Chemical Physics. 20 (3): 369–374. Bibcode:1952JChPh..20..369T. doi:10.1063/1.1700426.
- J. B. Conway; R. H. Wilson Jr.; A. V. Grosse (1953). "THE TEMPERATURE OF THE CYANOGEN-OXYGEN FLAME". Journal of the American Chemical Society. 75 (2): 499. doi:10.1021/ja01098a517.
External links
| Wikimedia Commons has media related to cyanogen. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Cyanogen. |