Peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is an unstable structural isomer of nitrate, NO−
3. Although its conjugate acid peroxynitrous acid is highly reactive, peroxynitrite is stable in basic solutions.[1][2] It is prepared by the reaction of hydrogen peroxide with nitrite:
- H2O2 + NO−
2 → ONOO− + H2O
 Chemical structure of the peroxynitrite anion | |
| Names | |
|---|---|
| IUPAC name
Oxido nitrite | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HNO3− | |
| Molar mass | 63.013 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
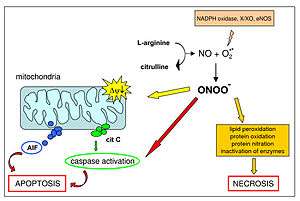
Peroxynitrite is an oxidant and nitrating agent. Because of its oxidizing properties, peroxynitrite can damage a wide array of molecules in cells, including DNA and proteins. Formation of peroxynitrite in vivo has been ascribed to the reaction of the free radical superoxide with the free radical nitric oxide:[3][4]
- O•−
2 + NO• → ONO−
2
The resultant pairing of these two free radicals results in peroxynitrite, a molecule that is itself not a free radical, but that is a powerful oxidant.
In the laboratory, a solution of peroxynitrite can be prepared by treating acidified hydrogen peroxide with a solution of sodium nitrite, followed by rapid addition of NaOH. Its concentration is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).[5]
As a nucleophile
ONOO− reacts nucleophilically with carbon dioxide. In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−
2) is by far the predominant pathway for ONOO−. ONOOCO−
2 homolyzes to form carbonate radical and nitrogen dioxide, again as a pair of caged radicals. Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate. The other 33% of the time, these two radicals escape the solvent cage and become free radicals. It is these radicals (carbonate radical and nitrogen dioxide) that are believed to cause peroxynitrite-related cellular damage.
Peroxynitrous acid
Peroxynitrous acid (HNO3) is a reactive nitrogen-containing species. It is the conjugate acid of peroxynitrite. It has a pKa of ~6.8.
See also
References
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Koppenol, W. H (1998). "The chemistry of peroxynitrite, a biological toxin<a name='TOP1'></a>". Química Nova. 21 (3): 326–331. doi:10.1590/S0100-40421998000300014.
- Pacher, P; Beckman, J. S; Liaudet, L (2007). "Nitric oxide and peroxynitrite in health and disease". Physiological Reviews. 87 (1): 315–424. doi:10.1152/physrev.00029.2006. PMC 2248324. PMID 17237348.
- Szabó, C; Ischiropoulos, H; Radi, R (2007). "Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics". Nature Reviews Drug Discovery. 6 (8): 662–80. doi:10.1038/nrd2222. PMID 17667957.
- Beckman, J. S; Koppenol, W. H (1996). "Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly". American Journal of Physiology. Cell Physiology. 271 (5 Pt 1): C1424–37. doi:10.1152/ajpcell.1996.271.5.C1424. PMID 8944624.