Nitrogen oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
- Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
- Nitrogen dioxide (NO2), nitrogen(IV) oxide
- Nitrogen trioxide (NO3), or nitrate radical, nitrogen(VI) oxide
- Nitrous oxide (N2O), nitrogen(0,II) oxide
- Dinitrogen dioxide (N2O2), nitrogen(II) oxide dimer
- Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide
- Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide dimer
- Dinitrogen pentoxide (N2O5), nitrogen(V) oxide, or nitronium nitrate [NO2]+[NO3]−
- Nitrosylazide (N4O), nitrogen(−I,0,I,II) oxide
- Oxatetrazole (N4O)
- Trinitramide (N(NO2)3 or N4O6), nitrogen(0,IV) oxide
Anions
- Nitroxide (NO−)
- Nitrite (NO−
2) - Nitrate (NO−
3) - Peroxynitrite (ONO−
2) - Peroxynitrate (ONO−
3) - Orthonitrate (NO3−
4) - Hyponitrite (N
2O2−
2) - Trioxodinitrate (N
2O2−
3), or hyponitrate - Nitroxylate (N
2O4−
4) - Dinitramide (N(NO
2)−
2 or N
3O−
4)
Cations
- Nitrosonium (NO+
) - Nitronium (NO+
2)
Atmospheric sciences
- NO
x (or NOx) refers to the sum of NO and NO2.[1][2] - NO
y (or NOy) = the sum of all oxidized atmospheric odd-nitrogen species (e.g. NOx + HNO3 + HNO2 + etc.) - NO
z (or NOz) = NOy − NOx
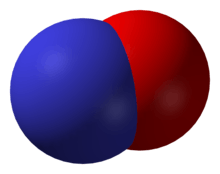
Nitric oxide, NO 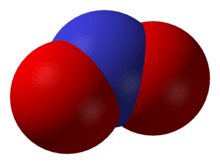
Nitrogen dioxide, NO2 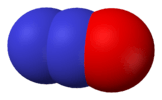
Nitrous oxide, N2O 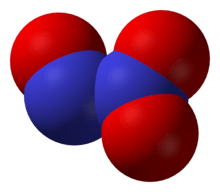
Dinitrogen trioxide, N2O3 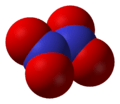
Dinitrogen tetroxide, N2O4 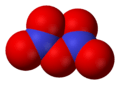
Dinitrogen pentoxide, N2O5 
Trinitramide, N4O6
gollark: Let's add this to the wiki.
gollark: esolangs IRC server but not the existing one when
gollark: Where's that from <@319753218592866315>? I kind of want to complain.
gollark: Discord ❤️ Other People's Open Source
gollark: https://osmarks.tk/wsthing/admin/reports, put in the key IgjuLVkKGYiyDIckqY_7
See also
- Nitrogen oxide sensor
- Sulfur nitrides, which are valence isoelectronic with nitrogen oxides
References
- United States Clean Air Act, 42 U.S.C. § 7602
- Seinfeld, John H.; Pandis, Spyros N. (1997), Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, Wiley-Interscience, ISBN 0-471-17816-0
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.