Kilogram
The kilogram (also kilogramme) is the base unit of mass in the metric system, formally the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo in everyday speech.
| kilogram | |
|---|---|
 | |
| General information | |
| Unit system | SI base unit |
| Unit of | mass |
| Symbol | kg |
| Conversions | |
| 1 kg in ... | ... is equal to ... |
| Avoirdupois | ≈ 2.205 pounds[Note 1] |
| British Gravitational | ≈ 0.0685 slugs |
The kilogram was originally defined in 1795 as the mass of one litre of water. This was a simple definition, but difficult to use in practice. By the latest definitions of the unit, however, this relationship still has an accuracy of 30 ppm. In 1799, the platinum Kilogramme des Archives replaced it as the standard of mass. In 1889, a cylinder of platinum-iridium, the International Prototype of the Kilogram (IPK) became the standard of the unit of mass for the metric system, and remained so until 2019.[1] The kilogram was the last of the SI units to be defined by a physical artefact.
The kilogram is now defined in terms of the second and the metre, based on fixed fundamental constants of nature.[2] This allows a properly-equipped metrology laboratory to calibrate a mass measurement instrument such as a Kibble balance as the primary standard to determine an exact kilogram mass, although the IPK and other precision kilogram masses remain in use as secondary standards for all ordinary purposes.
Definition
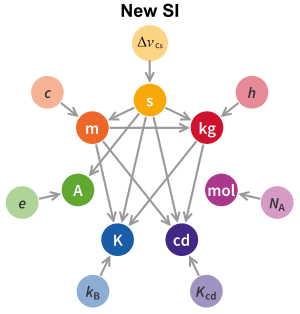
The kilogram is defined in terms of three fundamental physical constants: The speed of light c, a specific atomic transition frequency ΔνCs, and the Planck constant h. The formal definition is:
- The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.62607015×10−34 when expressed in the unit J⋅s, which is equal to kg⋅m2⋅s−1, where the metre and the second are defined in terms of c and ΔνCs.[3][4]
This definition makes the kilogram consistent with the older definitions: the mass remains within 30 ppm of the mass of one litre of water.[5]
Timeline of previous definitions
- 1793: The grave (the precursor of the kilogram) is defined as the mass of 1 litre (dm3) of water, which was determined to be 18841 grains.[6]
- 1795: the gram (1/1000 of a kilogram) was provisionally defined as the mass of one cubic centimetre of water at the melting point of ice.[7]
- 1799: The Kilogramme des Archives was manufactured as a prototype
- 1875-1889: The Metre Convention is signed in 1875, leading to production of The International Prototype of the Kilogram (IPK) in 1879 and its adoption in 1889. It had a mass equal to the mass of 1 dm3 of water under atmospheric pressure and at the temperature of its maximum density, which is approximately 4 °C.
- 2019-Present: the kilogram is currently redefined in terms of the Planck constant as approved by the General Conference on Weights and Measures (CGPM) on 16 November 2018.
Name and terminology
The kilogram is the only base SI unit with an SI prefix (kilo) as part of its name. The word kilogramme or kilogram is derived from the French kilogramme,[8] which itself was a learned coinage, prefixing the Greek stem of χίλιοι khilioi "a thousand" to gramma, a Late Latin term for "a small weight", itself from Greek γράμμα.[9] The word kilogramme was written into French law in 1795, in the Decree of 18 Germinal,[10] which revised the provisional system of units introduced by the French National Convention two years earlier, where the gravet had been defined as weight (poids) of a cubic centimetre of water, equal to 1/1000 of a grave.[11] In the decree of 1795, the term gramme thus replaced gravet, and kilogramme replaced grave.
The French spelling was adopted in Great Britain when the word was used for the first time in English in 1795,[12][8] with the spelling kilogram being adopted in the United States. In the United Kingdom both spellings are used, with "kilogram" having become by far the more common.[13] UK law regulating the units to be used when trading by weight or measure does not prevent the use of either spelling.[14]
In the 19th century the French word kilo, a shortening of kilogramme, was imported into the English language where it has been used to mean both kilogram[15] and kilometre.[16] While kilo as an alternative is acceptable, to The Economist for example,[17] the Canadian government's Termium Plus system states that "SI (International System of Units) usage, followed in scientific and technical writing" does not allow its usage and it is described as "a common informal name" on Russ Rowlett's Dictionary of Units of Measurement.[18][19] When the United States Congress gave the metric system legal status in 1866, it permitted the use of the word kilo as an alternative to the word kilogram,[20] but in 1990 revoked the status of the word kilo.[21]
The SI system was introduced in 1960, and in 1970 the BIPM started publishing the SI Brochure, which contains all relevant decisions and recommendations by the CGPM concerning units. The SI Brochure states that "It is not permissible to use abbreviations for unit symbols or unit names ...".[22][Note 2]
Kilogram becoming a base unit: the role of units for electromagnetism
As it happens, it is mostly because of units for electromagnetism that the kilogram rather than the gram was eventually adopted as the base unit of mass in the SI system. The relevant series of discussions and decisions started roughly in the 1850s and effectively concluded in 1946. In brief, by the end of the 19th century, the ‘practical units’ for electric and magnetic quantities such as the ampere and the volt were well established in practical use (e.g. for telegraphy). Unfortunately, they were not coherent with the then-prevailing base units for length and mass, the centimeter and the gram. However, the ‘practical units’ also included some purely mechanical units; in particular, the product of the ampere and the volt gives a purely mechanical unit of power, the watt. It was noticed that the purely mechanical practical units such as the watt would be coherent in a system in which the base unit of length was the meter and the base unit of mass was the kilogram. In fact, given that no one wanted to replace the second as the base unit of time, the meter and the kilogram are the only pair of base units of length and mass such that 1. the watt is a coherent unit of power, 2. the base units of length and time are decimal multiples or submultiples of the meter and the gram (so that the system remains ‘metric’), and 3. the sizes of the base units of length and mass are convenient for practical use.[Note 3] This would still leave out the purely electrical and magnetic units: while the purely mechanical practical units such as the watt are coherent in the meter-kilogram-second system, the explicitly electrical and magnetic units such as the volt, the ampere, etc. are not.[Note 5] The only way to also make those units coherent with the meter-kilogram-second system is to modify that system in a different way: one has to increase the number of fundamental dimensions from three (length, mass, and time) to four (the previous three, plus one purely electrical one).[Note 6]
The state of units for electromagnetism at the end of the 19th century
During the second half of the 19th century, the centimetre–gram–second (CGS) system of units was becoming widely accepted for scientific work, treating the gram as the fundamental unit of mass and the kilogram as a decimal multiple of the base unit formed by using a metric prefix. However, as the century drew to a close, there was widespread dissatisfaction with the state of units for electricity and magnetism in the CGS system. To begin with, there were two obvious choices for absolute units[Note 7] of electromagnetism: the ‘electrostatic’ (CGS-ESU) system and the ‘electromagnetic’ (CGS-EMU) system. But the main problem was that the sizes of coherent electric and magnetic units were not convenient in either of these systems; for example, the ESU unit of electrical resistance, which was later named the statohm, corresponds to about 9×1011 ohm, while the EMU unit, which was later named the abohm, corresponds to 10−9 ohm.[Note 8]
To circumvent this difficulty, a third set of units was introduced: the so-called practical units. The practical units were obtained as decimal multiples of coherent CGS-EMU units, chosen so that the resulting magnitudes were convenient for practical use and so that the practical units were, as far as possible, coherent with each other.[25] The practical units included such units as the volt, the ampere, the ohm, etc.,[26][27] which were later incorporated in the SI system and which we use to this day.[Note 9] Indeed, the main reason why the meter and the kilogram were later chosen to be the base units of length and mass was that they are the only combination of reasonably sized decimal multiples or submultiples of the meter and the gram that can in any way be made coherent with the volt, the ampere, etc.
The reason is that electrical quantities cannot be isolated from mechanical and thermal ones: they are connected by relations such as current × electric potential difference = power. For this reason, the practical system also included coherent units for certain mechanical quantities. For example, the previous equation implies that ampere × volt is a coherent derived practical unit of power;[Note 10] this unit was named the watt. The coherent unit of energy is then the watt times the second, which was named the joule. The joule and the watt also have convenient magnitudes and are decimal multiples of CGS coherent units for energy (the erg) and power (the erg per second). The watt is not coherent in the centimeter-gram-second system, but it is coherent in the meter-kilogram-second system—and in no other system whose base units of length and mass are reasonably sized decimal multiples or submultiples of the meter and the gram.
However, unlike the watt and the joule, the explicitly electrical and magnetic units (the volt, the ampere…) are not coherent even in the (absolute three-dimensional) meter-kilogram-second system. Indeed, one can work out what the base units of length and mass have to be in order for all the practical units to be coherent (the watt and the joule as well as the volt, the ampere, etc.). The values are 107 metres (one half of a meridian of the Earth, called a quadrant) and 10−11 grams (called an eleventh-gram[Note 11]).[Note 13]
Therefore, the full absolute system of units in which the practical electrical units are coherent is the quadrant–eleventh-gram–second (QES) system. However, the extremely inconvenient magnitudes of the base units for length and mass made it so that no one seriously considered adopting the QES system. Thus, people working on practical applications of electricity had to use units for electrical quantities and for energy and power that were not coherent with the units they were using for e.g. length, mass, and force.
Meanwhile, scientists developed a yet another fully coherent absolute system, which came to be called the Gaussian system, in which the units for purely electrical quantities are taken from CGE-ESU, while the units for magnetic quantities are taken from the CGS-EMU. This system proved very convenient for scientific work and is still widely used. However, the sizes of its units remained either too large or too small—by many orders of magnitude—for practical applications.
Finally, on top of all this, in both CGS-ESU and CGS-EMU as well as in the Gaussian system, Maxwell's equations are ‘unrationalized', meaning that they contain various factors of 4π that many workers found awkward. So yet another system was developed to rectify that: the ‘rationalized’ Gaussian system, usually called the Lorentz–Heaviside system. This system is still used in some subfields of physics. However, the units in that system are related to Gaussian units by factors of √4π ≈ 3.5, which means that their magnitudes remained, like those of the Gaussian units, either far too large or far too small for practical applications.
The Giorgi proposal
In 1901, Giovanni Giorgi proposed a new system of units that would remedy this state of affairs.[28] He noted that the mechanical practical units such as the joule and the watt are coherent not only in the QES system, but also in the meter-kilogram-second (MKS) system.[29][Note 14] It was of course known that just adopting the meter and the kilogram as base units—obtaining the three dimensional MKS system—would not solve the problem: while the watt and the joule would be coherent, this would not be so for the volt, the ampere, the ohm, and the rest of the practical units for electric and magnetic quantities (the only three-dimensional absolute system in which all practical units are coherent is the QES system).
But Giorgi pointed out that the volt and the rest could be made coherent if one gave up on the idea that all physical quantities must be expressible in terms of dimensions of length, mass, and time, and admitted a fourth base dimension for electric quantities. Any practical electrical unit could be chosen as the new fundamental unit, independent from the meter, kilogram, and second. Likely candidates for the fourth independed unit included the coulomb, the ampere, the volt, and the ohm, but eventually the ampere proved to be the most convenient as far as metrology. Moreover, the freedom gained by making an electric unit independent from the mechanical units could be used to rationalize Maxwell's equations.
The idea that one should give up on having a purely ‘absolute’ system (i.e. one where only length, mass, and time are the base dimensions) was a departure from a viewpoint that seemed to underlie the early breakthroughs by Gauss and Weber (especially their famous ‘absolute measurements' of Earth's magnetic field[30]:54–56), and it took some time for the scientific community to accept it—not least because many scientists clung to the notion that the dimensions of a quantity in terms of length, mass, and time somehow specify its ‘fundamental physical nature’.[31]:24, 26[29]
Acceptance of the Giorgi system, leading to the MKSA system and the SI
By the 1920s, dimensional analysis had become much better understood[29] and it was becoming widely accepted that the choice of both the number and of the identities of the fundamental dimensions should be dictated by convenience only and that there is nothing truly fundamental about the dimensions of a quantity.[31] In 1935, Giorgi's proposal was adopted by the IEC as the Giorgi system. It is this system that has since then been called the MKS system,[32] although ‘MKSA’ appears in careful usage. In 1946 the CIPM approved a proposal to adopt the ampere as the electromagnetic unit of the "MKSA system".[33]:109,110 In 1948 the CGPM commissioned the CIPM "to make recommendations for a single practical system of units of measurement, suitable for adoption by all countries adhering to the Metre Convention".[34] This led to the launch of SI in 1960.
To summarize, the ultimate reason why the kilogram was chosen over the gram as the base unit of length was, in one word, the volt-ampere. Namely, the combination of the meter and the kilogram was the only choice of base units of length and mass such that 1. the volt-ampere—which is also called the watt and which is the unit of power in the practical system of electrical units—is coherent, 2. the base units of length and time are decimal multiples or submultiples of the meter and the gram, and 3. the base units of length and time have convenient sizes.
The CGS and MKS systems co-existed during much of the early-to-mid 20th century, but as a result of the decision to adopt the "Giorgi system" as the international system of units in 1960, the kilogram is now the SI base unit for mass, while the definition of the gram is derived from that of the kilogram.
Redefinition based on fundamental constants

The replacement of the International Prototype of the Kilogram as primary standard was motivated by evidence accumulated over a long period of time that the mass of the IPK and its replicas had been changing; the IPK had diverged from its replicas by approximately 50 micrograms since their manufacture late in the 19th century. This led to several competing efforts to develop measurement technology precise enough to warrant replacing the kilogram artefact with a definition based directly on physical fundamental constants.[1] Physical standard masses such as the IPK and its replicas still serve as secondary standards.
The International Committee for Weights and Measures (CIPM) approved a redefinition of the SI base units in November 2018 that defines the kilogram by defining the Planck constant to be exactly 6.62607015×10−34 kg⋅m2⋅s−1, effectively defining the kilogram in terms of the second and the metre. The new definition took effect on 20 May 2019.[1][3][35]
Prior to the redefinition, the kilogram and several other SI units based on the kilogram were defined by a man-made metal artefact: the Kilogramme des Archives from 1799 to 1889, and the International Prototype of the Kilogram from 1889 onward.[1]
In 1960, the metre, previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by krypton,[36] and later the speed of light) so that the standard can be independently reproduced in different laboratories by following a written specification.
At the 94th Meeting of the International Committee for Weights and Measures (CIPM) in 2005, it was recommended that the same be done with the kilogram.[37]
In October 2010, the CIPM voted to submit a resolution for consideration at the General Conference on Weights and Measures (CGPM), to "take note of an intention" that the kilogram be defined in terms of the Planck constant, h (which has dimensions of energy times time, thus mass × length2 / time) together with other physical constants.[38][39] This resolution was accepted by the 24th conference of the CGPM[40] in October 2011 and further discussed at the 25th conference in 2014.[41][42] Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018.[41] Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The Kibble balance is one way to do this.
As part of this project, a variety of very different technologies and approaches were considered and explored over many years. Some of these approaches were based on equipment and procedures that would enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and which expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. All approaches depend on converting a weight measurement to a mass, and therefore require the precise measurement of the strength of gravity in laboratories. All approaches would have precisely fixed one or more constants of nature at a defined value.
SI multiples
Because SI prefixes may not be concatenated (serially linked) within the name or symbol for a unit of measure, SI prefixes are used with the unit gram, not kilogram, which already has a prefix as part of its name.[43] For instance, one-millionth of a kilogram is 1 mg (one milligram), not 1 μkg (one microkilogram).
| Submultiples | Multiples | |||||
|---|---|---|---|---|---|---|
| Value | SI symbol | Name | Value | SI symbol | Name | |
| 10−1 g | dg | decigram | 101 g | dag | decagram | |
| 10−2 g | cg | centigram | 102 g | hg | hectogram | |
| 10−3 g | mg | milligram | 103 g | kg | kilogram | |
| 10−6 g | µg | microgram | 106 g | Mg | megagram (tonne) | |
| 10−9 g | ng | nanogram | 109 g | Gg | gigagram | |
| 10−12 g | pg | picogram | 1012 g | Tg | teragram | |
| 10−15 g | fg | femtogram | 1015 g | Pg | petagram | |
| 10−18 g | ag | attogram | 1018 g | Eg | exagram | |
| 10−21 g | zg | zeptogram | 1021 g | Zg | zettagram | |
| 10−24 g | yg | yoctogram | 1024 g | Yg | yottagram | |
| Common prefixed units are in bold face.[Note 15] | ||||||
- The microgram is typically abbreviated "mcg" in pharmaceutical and nutritional supplement labelling, to avoid confusion, since the "μ" prefix is not always well recognised outside of technical disciplines.[Note 16] (The expression "mcg" is also the symbol for an obsolete CGS unit of measure known as the "millicentigram", which is equal to 10 μg.)
- In the United Kingdom, because serious medication errors have been made from the confusion between milligrams and micrograms when micrograms has been abbreviated, the recommendation given in the Scottish Palliative Care Guidelines is that doses of less than one milligram must be expressed in micrograms and that the word microgram must be written in full, and that it is never acceptable to use "mcg" or "μg".[44]
- The hectogram (100 g) is a very commonly used unit in the retail food trade in Italy, usually called an etto, short for ettogrammo, the Italian for hectogram.[45][46][47]
- The former standard spelling and abbreviation "deka-" and "dk" produced abbreviations such as "dkm" (dekametre) and "dkg" (dekagram).[48] As of 2020, the abbreviation "dkg" (10 g) is still used in parts of central Europe in retail for some foods such as cheese and meat, e.g. here:[49][50][51][53].
- The unit name megagram is rarely used, and even then typically only in technical fields in contexts where especially rigorous consistency with the SI standard is desired. For most purposes, the name tonne is instead used. The tonne and its symbol, "t", were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. According to the BIPM, "This unit is sometimes referred to as 'metric ton' in some English-speaking countries."[54] The unit name megatonne or megaton (Mt) is often used in general-interest literature on greenhouse gas emissions, whereas the equivalent unit in scientific papers on the subject is often the teragram (Tg).
See also
- 1795 in science
- 1799 in science
- General Conference on Weights and Measures (CGPM)
- Gram
- Grave (orig. name of the kilogram, history of)
- Gravimetry
- Inertia
- International Bureau of Weights and Measures (BIPM)
- International Committee for Weights and Measures (CIPM)
- International System of Units (SI)
- Kibble balance
- Kilogram-force
- Litre
- Mass
- Mass versus weight
- Metric system
- Metric ton
- Milligram per cent
- National Institute of Standards and Technology (NIST)
- Newton
- SI base units
- Standard gravity
- Weight
Notes
- The avoirdupois pound is part of both United States customary system of units and the Imperial system of units. It is defined as exactly 0.45359237 kilograms.
- The French text (which is the authoritative text) states "Il n'est pas autorisé d'utiliser des abréviations pour les symboles et noms d'unités ..."
- Let us show that, if it is known that the meter and the kilogram satisfy all three conditions, then no other choice does. The coherent unit of power, when written out in terms of the base units of length, mass, and time, is (base unit of mass) × (base unit of length)2/(base unit of time)3. We are told that the watt is coherent in the meter-kilogram-second system; thus, 1 watt = (1 kg) × (1 m)2/(1 s)3. We will leave the second as is, and note that if the base unit of length is changed to L m and the base unit of mass to M kg, then the coherent unit of power is (M kg) × (L m)2/(1 s)3 = ML2 × (1 kg) × (1 m)2/(1 s)3 = ML2 watt. Since we are looking for base units of length and mass such that the coherent unit of power is the watt, we require that ML2 = 1. It follows that if we change the base unit of length by a factor of L, then we must change the base unit of mass by a factor of 1/L2 if the watt is to remain a coherent unit. It would be impractical to make the base unit of length a decimal multiple of a meter (10 m, 100 m, or more). Therefore our only option is to make the base unit of length a decimal submultiple of the meter. This would mean decreasing the meter by a factor of 10 to obtain the decimeter (0.1 m), or by a factor of 100 to get the centimeter, or by a factor of 1000 to get the millimeter. Making the base unit of length even smaller would not be practical (for example, the next decimal factor, 10000, would produce the base unit of length of one-tenth of a millimeter), so these three factors (10, 100, and 1000) are the only acceptable options as far as the base unit of length. But then the base unit of mass would have to be larger than a kilogram, by the following respective factors: 102 = 100, 1002 = 10000, and 10002 = 106. In other words, the watt is a coherent unit for the following pairs of base units of length and mass: 0.1 m and 100 kg, 1 cm and 10000 kg, and 1 mm and 1000000 kg. Even in the first pair, the base unit of mass is impractically large, 100 kg, and as the base unit of length is decreased, the base unit of mass gets even larger. Thus, assuming that the second remains the base unit of time, the meter-kilogram combination is the only one such that the base units for both length and mass are neither too large nor too small, and such that they are decimal multiples or submultiples of the meter and the gram, and such that the watt is a coherent unit.
- A system in which the base quantities are length, mass, and time, and only those three.
- We will see that there is only one three-dimensional ‘absolute’ system[Note 4] in which all practical units are coherent, including the volt, the ampere, etc.: one in which the base unit of length is 107 m and the base unit of mass is 10−11 g. Clearly, these magnitudes are not practical.
- Meanwhile, there were parallel developments that, for independent reasons, eventually resulted in three additional fundamental dimensions, for a total of seven: those for temperature, luminous intensity, and the amount of substance.
- That is, units that have length, mass, and time as base dimensions and that are coherent in the CGS system.
- For quite a long time, the ESU and EMU units didn't have special names; one would just say, e.g. the ESU unit of resistance. It was apparently only in 1903 that A. E. Kennelly suggested that the names of the EMU units be obtained by prefixing the name of the corresponding ‘practical unit' by ‘ab-’ (short for ‘absolute’, giving the ‘abohm’, ‘abvolt’, the ‘abampere’, etc.), and that the names of the ESU units be analogously obtained by using the prefix ‘abstat-’, which was later shortened to ‘stat-’ (giving the ‘statohm’, ‘statvolt’, ‘statampere’, etc.).[23]:534–5 This naming system was widely used in the U.S., but, apparently, not in Europe.[24]
- The use of SI electrical units is essentially universal worldwide (besides the clearly electrical units like the ohm, the volt, and the ampere, it is also nearly universal to use the watt when quantifying specifically electrical power). This is so even in the United States and the United Kingdom, two notable countries that are among a handful of nations that, to various degrees, continue to resist widespread internal adoption of the SI system. But the resistance to the adoption of SI units mostly concerns mechanical units (lengths, mass, force, torque, pressure), thermal units (temperature, heat), and units for describing ionizing radiation (activity referred to a radionuclide, absorbed dose, dose equivalent); it does not concern electrical units.
- In alternating current (AC) circuits one can introduce three kinds of power: active, reactive, and apparent. Though the three have the same dimensions and thus the same units when those are expressed in terms of base units (i.e. kg⋅m2⋅s-3), it is customary to use different names for each: respectively, the watt, the volt-ampere reactive, and the volt-ampere.
- At the time, it was popular to denote decimal multiples and submultiples of quantities by using a system suggested by G. J. Stoney. The system is easiest to explain through examples. For decimal multiples: 109 grams would be denoted as gram-nine, 1013 m would be a meter-thirteen, etc. For submultiples: 10−9 grams would be denoted as a ninth-gram, 10−13 m would be a thirteenth-meter, etc. The system also worked with units that used metric prefixes, so e.g. 1015 centimeter would be centimeter-fifteen. The rule, when spelled out, is this: we denote ‘the exponent of the power of 10, which serves as multiplier, by an appended cardinal number, if the exponent be positive, and by a prefixed ordinal number, if the exponent be negative.’[26]
- This is also obvious from the fact that in both absolute and practical units, current is charge per unit time, so that the unit of time is the unit of charge divided by the unit of current. In the practical system, we know that the base unit of time is the second, so the coulomb per ampere gives the second. The base unit of time in CGS-EMU is then the abcoulomb per abampere, but that ratio is the same as the coulomb per ampere, since the units of current and charge both use the same conversion factor, 0.1, to go between the EMU and practical units (coulomb/ampere = (0.1 abcoulomb)/(0.1 abampere) = abcoulomb/abampere). So the base unit of time in EMU is also the second.
- This can be shown from the definitions of, say, the volt, the ampere, and the coulomb in terms of the EMU units. The volt was chosen as 108 EMU units (abvolts), the ampere as 0.1 EMU units (abamperes), and the coulomb as 0.1 EMU units (abcoulombs). Now we use the fact that, when expressed in the base CGS units, the abvolt is g1/2·cm3/2/s2, the abampere is g1/2·cm1/2/s, and the abcoulomb is g1/2·cm1/2. Suppose we choose new base units of length, mass, and time, equal to L centimeters, M grams, and T seconds. Then instead of the abvolt, the unit of electric potential will be (M × g)1/2·(L × cm)3/2/(T × s)2 = M1/2L3/2/T2 × g1/2·cm3/2/s2 = M1/2L3/2/T2 abvolts. We want this new unit to be the volt, so we must have M1/2L3/2/T2 = 108. Similarly, if we want the new unit for current to be the ampere, we obtain that M1/2L1/2/T = 0.1, and if we want the new unit of charge to be the coulomb, we get that M1/2L1/2 = 0.1. This is a system of three equations with three unknowns. By dividing the middle equation by the last one, we get that T = 1, so the second should remain the base unit of time.[Note 12] If we then divide the first equation by the middle one (and use the fact that T = 1), we get that L = 108/0.1 = 109, so the base unit of length should be 109 cm = 107 m. Finally, we square the final equation and obtain that M = 0.12/L = 10−11, so the base unit of mass should be 10−11 grams.
- To see this, we first note that the dimensions of energy are ML2/T2 and of power, ML2/T3. One meaning of these dimensional formulas is that if the unit of mass is changed by a factor of M, the unit of length by a factor of L, and the unit of time by a factor of T, then the unit of energy will change by a factor of ML2/T2 and the unit of power by a factor of ML2/T3. This means that if decrease the unit of length while at the same time increasing the unit of mass in such a way that the product ML2 remains constant, the units of energy and power would not change. Clearly, this happens if M = 1/L2. Now, we know that the watt and joule are coherent in a system in which the base unit of length is 107 m while the base unit of mass is 10−11 grams. We have just learned that they will then also be coherent in any system in which the base unit of length is L × 107 m and the base unit of mass is 1/L2 × 10−11 g, where L is any positive real number. If we set L = 10−7, we obtain the meter as the base unit of length. Then the corresponding base unit of mass works out to be 1/(10−7)2 × 10−11 g=1014 × 10−11 g = 103 g = 1 kg.
- Criterion: A combined total of at least five occurrences on the British National Corpus and the Corpus of Contemporary American English, including both the singular and the plural for both the -gram and the -gramme spelling.
- The practice of using the abbreviation "mcg" rather than the SI symbol "μg" was formally mandated in the US for medical practitioners in 2004 by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) in their "Do Not Use" List: Abbreviations, Acronyms, and Symbols because "μg" and "mg" when handwritten can be confused with one another, resulting in a thousand-fold overdosing (or underdosing). The mandate was also adopted by the Institute for Safe Medication Practices.
References
- Resnick, Brian (May 20, 2019). "The new kilogram just debuted. It's a massive achievement". vox.com. Retrieved May 23, 2019.
- "The Latest: Landmark Change to Kilogram Approved". AP News. Associated Press. November 16, 2018. Retrieved March 4, 2020.
- Draft Resolution A "On the revision of the International System of units (SI)" to be submitted to the CGPM at its 26th meeting (2018) (PDF)
- Decision CIPM/105-13 (October 2016). The day is the 144th anniversary of the Metre Convention.
- The density of water is 0.999972 g/cm3 at 3.984 °C. See Franks, Felix (2012). The Physics and Physical Chemistry of Water. Springer. ISBN 978-1-4684-8334-5.
- Guyton; Lavoisier; Monge; Berthollet; et al. (1792). Annales de chimie ou Recueil de mémoires concernant la chimie et les arts qui en dépendent. 15-16. Paris: Chez Joseph de Boffe. p. 277.
- Gramme, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante
- "Kilogram". Oxford English Dictionary. Oxford University Press. Retrieved November 3, 2011.
- Fowlers, HW; Fowler, FG (1964). The Concise Oxford Dictionary. Oxford: The Clarendon Press. Greek γράμμα (as it were γράφ-μα, Doric γράθμα) means "something written, a letter", but it came to be used as a unit of weight, apparently equal to 1/24 of an ounce (1/288 of a libra, which would correspond to about 1.14 grams in modern units), at some time during Late Antiquity. French gramme was adopted from Latin gramma, itself quite obscure, but found in the Carmen de ponderibus et mensuris (8.25) attributed by Remmius Palaemon (fl. 1st century), where it is the weight of two oboli (Charlton T. Lewis, Charles Short, A Latin Dictionary s.v. "gramma", 1879). Henry George Liddell. Robert Scott. A Greek-English Lexicon (revised and augmented edition, Oxford, 1940) s.v. γράμμα, citing the 10th-century work Geoponica and a 4th-century papyrus edited in L. Mitteis, Griechische Urkunden der Papyrussammlung zu Leipzig, vol. i (1906), 62 ii 27.
- "Décret relatif aux poids et aux mesures du 18 germinal an 3 (7 avril 1795)" [Decree of 18 Germinal, year III (April 7, 1795) regarding weights and measures]. Grandes lois de la République (in French). Digithèque de matériaux juridiques et politiques, Université de Perpignan. Retrieved November 3, 2011.
- Convention nationale, décret du 1er août 1793, ed. Duvergier, Collection complète des lois, décrets, ordonnances, règlemens avis du Conseil d'état, publiée sur les éditions officielles du Louvre, vol. 6 (2nd ed. 1834), p. 70. The metre (mètre) on which this definition depends was itself defined as the ten-millionth part of a quarter of Earth's meridian, given in traditional units as 3 pieds, 11.44 lignes (a ligne being the 12th part of a pouce (inch), or the 144th part of a pied.
- Peltier, Jean-Gabriel (1795). "Paris, during the year 1795". Monthly Review. 17: 556. Retrieved August 2, 2018. Contemporaneous English translation of the French decree of 1795
- "Kilogram". Oxford Dictionaries. Archived from the original on January 31, 2013. Retrieved November 3, 2011.
- "Spelling of "gram", etc". Weights and Measures Act 1985. Her Majesty's Stationery Office. October 30, 1985. Retrieved November 6, 2011.
- "kilo (n1)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved November 8, 2011.
- "kilo (n2)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved November 8, 2011.
- "Style Guide" (PDF). The Economist. January 7, 2002. Archived from the original (PDF) on July 1, 2017. Retrieved November 8, 2011.
- "kilogram, kg, kilo". Termium Plus. Government of Canada. October 8, 2009. Retrieved May 29, 2019.
- "kilo". How Many?. Archived from the original on November 16, 2011. Retrieved November 6, 2011.
- 29th Congress of the United States, Session 1 (May 13, 1866). "H.R. 596, An Act to authorize the use of the metric system of weights and measures". Archived from the original on July 5, 2015.
- "Metric System of Measurement:Interpretation of the International System of Units for the United States; Notice" (PDF). Federal Register. 63 (144): 40340. July 28, 1998. Archived from the original (PDF) on October 15, 2011. Retrieved November 10, 2011.
Obsolete Units As stated in the 1990 Federal Register notice, ...
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 130, ISBN 92-822-2213-6, archived (PDF) from the original on August 14, 2017
- Kennelly, A. E. (July 1903). "Magnetic Units and Other Subjects that Might Occupy Attention at the Next International Electrical Congress". Transactions of the American Institute of Electrical Engineers. XXII: 529–536. doi:10.1109/T-AIEE.1903.4764390.
[p. 534] The expedient suggests itself of attaching the prefix ab or abs to a practical or Q. E. S. unit, in order to express the absolute or corresponding C. G. S. magnetic unit. … [p. 535] In a comprehensive system of electromagnetic terminology, the electric C. G. S. units should also be christened. They are sometimes referred to in electrical papers, but always in an apologetic, symbolical fashion, owing to the absence of names to cover their nakedness. They might be denoted by the prefix abstat.
- Silsbee, Francis (April–June 1962). "Systems of Electrical Units". Journal of Research of the National Bureau of Standards Section C. 66C (2): 137–183. doi:10.6028/jres.066C.014.
- "Units, Physical". Encyclopædia Britannica. 27 (11th ed.). New York : Encyclopaedia Britannica. 1911. p. 740.
- Thomson, Sir W.; Foster, C. G.; Maxwell, J. C.; Stoney, G. J.; Jenkin, Fleeming; Siemens; Bramwell, F. J.; Everett (1873). Report of the 43rd Meeting of the British Association for the Advancement of Science. Bradford. p. 223.}
- "The Electrical Congress". The Electrician. 7: 297. September 24, 1881. Retrieved June 3, 2020.
- Giovanni Giorgi (1901), "Unità Razionali di Elettromagnetismo", Atti della Associazione Elettrotecnica Italiana (in Italian), Torino, OL 18571144M Giovanni Giorgi (1902), Rational Units of Electromagnetism Original manuscript with handwritten notes by Oliver Heaviside
- Giorgi, Giovanni (2018) [Originally published in June, 1934 by the Central Office of the International Electrotechnical Commission (IEC), London, for IEC Advisory Committee No. 1 on Nomenclature, Section B: Electric and Magnetic Magnitudes and Units.]. "Memorandum on the M.K.S. System of Practical Units". IEEE Magnetics Letters. 9: 1–6. doi:10.1109/LMAG.2018.2859658.
- Carron, Neal (2015). "Babel of Units. The Evolution of Units Systems in Classical Electromagnetism". arXiv:1506.01951 [physics.hist-ph].
- Bridgman, P. W. (1922). Dimensional Analysis. Yale University Press.
- Arthur E. Kennelly (1935), "Adoption of the Meter–Kilogram–Mass–Second (M.K.S.) Absolute System of Practical Units by the International Electrotechnical Commission (I.E.C.), Bruxelles, June, 1935", Proceedings of the National Academy of Sciences of the United States of America, 21 (10): 579–583, Bibcode:1935PNAS...21..579K, doi:10.1073/pnas.21.10.579, PMC 1076662, PMID 16577693
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), ISBN 92-822-2213-6, archived (PDF) from the original on August 14, 2017
- Resolution 6 – Proposal for establishing a practical system of units of measurement. 9th Conférence Générale des Poids et Mesures (CGPM). October 12–21, 1948. Retrieved May 8, 2011.
- Pallab Ghosh (November 16, 2018). "Kilogram gets a new definition". BBC News. Retrieved November 16, 2018.
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 112, ISBN 92-822-2213-6, archived (PDF) from the original on August 14, 2017
- Recommendation 1: Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants (PDF). 94th meeting of the International Committee for Weights and Measures. October 2005. p. 233. Archived (PDF) from the original on June 30, 2007. Retrieved February 7, 2018.
- "NIST Backs Proposal for a Revamped System of Measurement Units". Nist.gov. October 26, 2010. Retrieved April 3, 2011.
- Ian Mills (September 29, 2010). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). CCU. Retrieved January 1, 2011.
- Resolution 1 – On the possible future revision of the International System of Units, the SI (PDF). 24th meeting of the General Conference on Weights and Measures. Sèvres, France. October 17–21, 2011. Retrieved October 25, 2011.
- "BIPM - Resolution 1 of the 25th CGPM". www.bipm.org. Retrieved March 27, 2017.
- "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. October 23, 2011. Retrieved October 25, 2011.
- BIPM: SI Brochure: Section 3.2, The kilogram
- "Prescribing Information for Liquid Medicines". Scottish Palliative Care Guidelines. Archived from the original on July 10, 2018. Retrieved June 15, 2015.
- Tom Stobart, The Cook's Encyclopedia, 1981, p. 525
- J.J. Kinder, V.M. Savini, Using Italian: A Guide to Contemporary Usage, 2004, ISBN 0521485568, p. 231
- Giacomo Devoto, Gian Carlo Oli, Nuovo vocabolario illustrato della lingua italiana, 1987, s.v. 'ètto': "frequentissima nell'uso comune: un e. di caffè, un e. di mortadella; formaggio a 2000 lire l'etto"
- U.S. National Bureau of Standards, The International Metric System of Weights and Measures, "Official Abbreviations of International Metric Units", 1932, p. 13
- "Jestřebická hovězí šunka 10 dkg | Rancherské speciality". eshop.rancherskespeciality.cz (in Czech). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- "Sedliacka šunka 1 dkg | Gazdovský dvor - Farma Busov Gaboltov". Sedliacka šunka 1 dkg (in Slovak). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- "sýr bazalkový - Farmářské Trhy". www.e-farmarsketrhy.cz (in Czech). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- "Termékek – Csíz Sajtműhely" (in Hungarian). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- Non-SI units that are accepted for use with the SI, SI Brochure: Section 4 (Table 8), BIPM
External links
| Wikimedia Commons has media related to Kilogram. |
- NIST Improves Accuracy of 'Watt Balance' Method for Defining the Kilogram
- The UK's National Physical Laboratory (NPL): Are any problems caused by having the kilogram defined in terms of a physical artefact? (FAQ - Mass & Density)
- NPL: NPL Kibble balance
- Metrology in France: Watt balance
- Australian National Measurement Institute: Redefining the kilogram through the Avogadro constant
- International Bureau of Weights and Measures (BIPM): Home page
- NZZ Folio: What a kilogram really weighs
- NPL: What are the differences between mass, weight, force and load?
- BBC: Getting the measure of a kilogram
- NPR: This Kilogram Has A Weight-Loss Problem, an interview with National Institute of Standards and Technology physicist Richard Steiner
- Avogadro and molar Planck constants for the redefinition of the kilogram
- Realization of the awaited definition of the kilogram
- Sample, Ian (November 9, 2018). "In the balance: scientists vote on first change to kilogram in a century". The Guardian. Retrieved November 9, 2018.
