Dinitrogen pentoxide
Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature,[1] yielding a colorless gas.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dinitrogen pentaoxide | |
| Other names
Nitric anhydride Nitronium nitrate Nitryl nitrate DNPO Anhydrous nitric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.227 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N2O5 | |
| Molar mass | 108.01 g/mol |
| Appearance | white solid |
| Density | 1.642 g/cm3 (18 °C) |
| Melting point | 41 °C (106 °F; 314 K) [1] |
| Boiling point | 47 °C (117 °F; 320 K) sublimes |
| reacts to give HNO3 | |
| Solubility | soluble in chloroform negligible in CCl4 |
| −35.6·10−6 cm3/mol (aq) | |
| 1.39 D | |
| Structure | |
| hexagonal | |
| planar, C2v (approx. D2h) N–O–N ≈ 180° | |
| Thermochemistry | |
Std molar entropy (S |
178.2 J K−1 mol−1 (s) 355.6 J K−1 mol−1 (g) |
Std enthalpy of formation (ΔfH⦵298) |
−43.1 kJ/mol (s) +11.3 kJ/mol (g) |
Gibbs free energy (ΔfG˚) |
114.1 kJ/mol |
| Hazards | |
| Main hazards | strong oxidizer, forms strong acid in contact with water |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
| Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen tetroxide | |
Related compounds |
Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dinitrogen pentoxide is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by NO2BF4 (nitronium tetrafluoroborate).
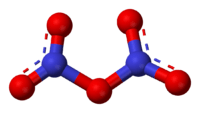
N2O5 is a rare example of a compound that adopts two structures depending on the conditions. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations [NO2]+ and nitrate anions [NO3]−; but in the gas phase and under some other conditions it is a covalently bound molecule.[3]
History
N2O5 was first reported by Deville in 1840, who prepared it by treating AgNO3 with Cl2.[4][5]
Structure and physical properties
Pure solid N2O5 is a salt, consisting of separated linear nitronium ions NO2+ and planar trigonal nitrate anions NO3−. Both nitrogen centers have oxidation state +5. It crystallizes in the space group D46h (C6/mmc) with Z = 2, with the NO−
3 anions in the D3h sites and the NO+
2 cations in D3d sites.[6]
The vapor pressure P (in torr) as a function of temperature T(in kelvin), in the range 211 to 305 K, is well approximated by the formula
being about 48 torr at 0 °C, 424 torr at 25 °C, and 760 torr at 32 °C (9 degrees below the melting point).[7]
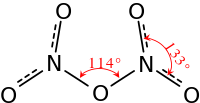
In the gas phase, or when dissolved in a nonpolar solvents such as CCl4, the compound exists as covalently bound molecules O2N–O–NO2. In the gas phase, theoretical calculations for the minimum-energy configuration indicate that the O–N–O angle in each NO
2 wing is about 134° and the N–O–N angle is about 112°. In that configuration, the two NO
2 groups are rotated about 35° around the bonds to the central oxygen, away from the N–O–N plane. The molecule thus has a propeller shape, with one axis of 180° rotational symmetry (C2) [8]
When gaseous N
2O
5 is cooled rapidly ("quenched"), one can obtain the metastable molecular form, which exothermically converts to the ionic form above −70 °C.[9]
Gaseous N
2O
5 absorbs ultraviolet light with dissociation into the radicals nitrogen dioxide NO
2 and nitrogen trioxide NO
3 (uncharged nitrate). The absorption spectrum has a broad band with maximum at wavelength 160 nm.[10]
Preparation
A recommended laboratory synthesis entails dehydrating nitric acid (HNO3) with phosphorus(V) oxide:[9]
- P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5
Another laboratory process is the reaction of lithium nitrate LiNO
3 and bromine pentafluoride BrF
5, in the ratio exceeding 3:1. The reaction first forms nitryl flouride FNO
2 that reacts further with the lithium nitrate:[6]
- BrF
5 + 3LiNO
3 → 3LiF + BrONO
2 + O2 + 2FNO2 - FNO2 + LiNO
3 → LiF + N
2O
5
The compound can also be created in the gas phase by reacting nitrogen dioxide NO
2 or N
2O
4 with ozone:[11]
- 2NO
2 + O
3 → N
2O
5 + O
2
However, the product catalyzes the rapid decomposition of ozone:[11]
- 2O
3 + N
2O
5 → 3O
2 + N
2O
5
Dinitrogen pentoxide is also formed when a mixture of oxygen and nitrogen is passed through an electric
discharge.[6] Another route is the reactions of POCl
3 or NO
2Cl with AgNO
3[6]
Reactions
Dinitrogen pentoxide reacts with water (hydrolyses) to produce nitric acid HNO
3. Thus, dinitrogen pentoxide is the anhydride of nitric acid:[9]
- N2O5 + H2O → 2 HNO
3
Solutions of dinitrogen pentoxide in nitric acid can be seen as nitric acid with more than 100% concentration. The phase diagram of the system H
2O−N
2O
5 shows the well-known negative azeotrope at 60% N
2O
5 (that is, 70% HNO
3), a positive azeotrope at 85.7% N
2O
5 (100% HNO
3), and another negative one at 87.5% N
2O
5 ("102% HNO
3").[12]
The reaction with hydrogen chloride HCl also gives nitric acid and nitrosym chlorideNO
2Cl:[13]
- N
2O
5 + HCl → HNO
3 + NO
2Cl
Dinitrogen pentoxide eventually decomposes at room temperature into NO2 and O2.[14][11] Decomposition is negligible if the solid is kept at 0 °C, in suitably inert containers.[6]
Dinitrogen pentoxide reacts with ammonia NH
3 to give several products, including nitrous oxide N
2O, ammonium nitrate NH
4NO
3, nitramide NH
2NO
2 and ammonium dinitramide NH
4N(NO
2)
2, depending on reaction condiitons.[15]
Applications
Nitration of organic compounds
Dinitrogen pentoxide, for example as a solution in chloroform, has been used as a reagent to introduce the NO2 functionality in organic compounds. This nitration reaction is represented as follows:
- N2O5 + Ar–H → HNO3 + Ar–NO2
where Ar represents an arene moiety.[16] The reactivity of the NO2+ can be further enhanced with strong acids that generate the "super-electrophile" HNO22+.
In this use, N
2O
5 has been largely replaced by nitronium tetrafluoroborate [NO
2]+[BF
4]−. This salt retains the high reactivity of NO2+, but it is thermally stable, decomposing at about 180 °C (into NO2F and BF3).
Dinitrogen pentoxide is relevant to the preparation of explosives.[5][17]
Atmospheric occurrence
In the atmosphere, dinitrogen pentoxide is an important reservoir of the NOx species that are responsible for ozone depletion: its formation provides a null cycle with which NO and NO2 are temporarily held in an unreactive state.[18] Mixing ratios of several ppbv have been observed in polluted regions of the night-time troposphere.[19] Dinitrogen pentoxide has also been observed in the stratosphere[20] at similar levels, the reservoir formation having been postulated in considering the puzzling observations of a sudden drop in stratospheric NO2 levels above 50 °N, the so-called 'Noxon cliff'.
Variations in N2O5 reactivity in aerosols can result in significant losses in tropospheric ozone, hydroxyl radicals, and NOx concentrations.[21] Two important reactions of N2O5 in atmospheric aerosols are: 1) Hydrolysis to form nitric acid[22] and 2) Reaction with halide ions, particularly Cl−, to form ClNO2 molecules which may serve as precursors to reactive chlorine atoms in the atmosphere.[23][24]
Hazards
N2O5 is a strong oxidizer that forms explosive mixtures with organic compounds and ammonium salts. The decomposition of dinitrogen pentoxide produces the highly toxic nitrogen dioxide gas.
References
- Emeleus (1 January 1964). Advances in Inorganic Chemistry. Academic Press. pp. 77–. ISBN 978-0-12-023606-0. Retrieved 20 September 2011.
- Peter Steele Connell The Photochemistry of Dinitrogen Pentoxide. Ph. D. thesis, Lawrence Berkeley National Laboratory.
- W. Rogie Angus, Richard W. Jones, and Glyn O. Phillips (1949): "Existence of Nitrosyl Ions (NO+
) in Dinitrogen Tetroxide and of Nitronium Ions (NO+
2) in Liquid Dinitrogen Pentoxide". Nature, volume 164, pages 433–434. doi:10.1038/164433a0 - M.H. Deville (1849). "Note sur la production de l'acide nitrique anhydre". Compt. Rend. 28: 257–260.
- Jai Prakash Agrawal (19 April 2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley-VCH. pp. 117–. ISBN 978-3-527-32610-5. Retrieved 20 September 2011.
- William W. Wilson and Karl O. Christe (1987): "Dinitrogen Pentoxide. New Synthesis and Laser Raman Spectrum". Inorganic Chemistry, volume 26, pages 1631-1633. doi:10.1021/ic00257a033
- A. H. McDaniel, J. A. Davidson, C. A. Cantrell, R. E. Shetter, and J. G. Calvert (1988): "Enthalpies of formation of dinitrogen pentoxide and the nitrate free radical". Journal of Physical Chemistry, volume 92, issue 14, pages 4172-4175. doi:10.1021/j100325a035
- S. Parthiban, B. N. Raghunandan, and R.Sumathi (1996): "Structures, energies and vibrational frequencies of dinitrogen pentoxide". Journal of Molecular Structure: THEOCHEM, volume 367, pages 111-118. doi:10.1016/S0166-1280(96)04516-2
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- Bruce A. Osborne, George Marston, L. Kaminski, N. C. Jones, J. M. Gingell, Nigel Mason, Isobel C. Walker, J. Delwiche, and M.-J. Hubin-Franskin (2000): "Vacuum ultraviolet spectrum of dinitrogen pentoxide". Journal of Quantitative Spectroscopy and Radiative Transfer, volume 64, issue 1, pages 67-74. doi:10.1016/S0022-4073(99)00104-1
- Francis Yao, Ivan Wilson, and Harold Johnston (1982): "Temperature-dependent ultraviolet absorption spectrum for dinitrogen pentoxide". Journal of Physical Chemistry, volume 86, issue 18, pages 3611-3615. doi:10.1021/j100215a023
- L. Lloyd and P. A. H. Wyatt (1955): "The vapour pressures of nitric acid solutions. Part I. New azeotropes in the water–dinitrogen pentoxide system". Journal of the Chemical Society (Resumed), volume 1955, pages 2248-2252.doi:10.1039/JR9550002248
- Robert A. Wilkins Jr. and I. C. Hisatsune (1976): "The Reaction of Dinitrogen Pentoxide with Hydrogen Chloride". Industrial & Engineering Chemistry Fundamentals, volume 15, issue 4, pages 246-248. doi:10.1021/i160060a003
- Nitrogen(V) Oxide. Inorganic Syntheses. 3. 1950. pp. 78–81.
- C. Frenck and W. Weisweiler (2002): "Modeling the Reactions Between Ammonia and Dinitrogen Pentoxide to Synthesize Ammonium Dinitramide (ADN)". Chemical Engineering & Technology, volume 25, issue 2, pages 123-128. doi:10.1002/1521-4125(200202)25:2<123::AID-CEAT123>3.0.CO;2-W
- Jan M. Bakke and Ingrd Hegbom (1994): "Dinitrogen pentoxide-sulfur dioxide, a new nitration system". Acta chemica scandinavica, volume 48, issue 2, pages 181-182. doi:10.3891/acta.chem.scand.48-0181
- Talawar, M. B.; et al. (2005). "Establishment of Process Technology for the Manufacture of Dinitrogen Pentoxide and its Utility for the Synthesis of Most Powerful Explosive of Today—CL-20". Journal of Hazardous Materials. 124 (1–3): 153–64. doi:10.1016/j.jhazmat.2005.04.021. PMID 15979786.
- Finlayson-Pitts, Barbara J.; Pitts, James N. (2000). Chemistry of the upper and lower atmosphere : theory, experiments, and applications. San Diego: Academic Press. ISBN 9780080529073. OCLC 162128929.
- HaiChao Wang; et al. (2017). "High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway". Environmental Science and Technology Letters. 4 (10): 416–420. doi:10.1021/acs.estlett.7b00341.
- C.P. Rinsland; et al. (1989). "Stratospheric N205 profiles at sunrise and sunset from further analysis of the ATMOS/Spacelab 3 solar spectra". Journal of Geophysical Research. 94: 18341–18349. Bibcode:1989JGR....9418341R. doi:10.1029/JD094iD15p18341.
- Macintyre, H. L.; Evans, M. J. (2010-08-09). "Sensitivity of a global model to the uptake of N2O5 by tropospheric aerosol". Atmospheric Chemistry and Physics. 10 (15): 7409–7414. doi:10.5194/acp-10-7409-2010. ISSN 1680-7324.
- Brown, S. S.; Dibb, J. E.; Stark, H.; Aldener, M.; Vozella, M.; Whitlow, S.; Williams, E. J.; Lerner, B. M.; Jakoubek, R. (2004-04-16). "Nighttime removal of NOx in the summer marine boundary layer". Geophysical Research Letters. 31 (7): n/a. doi:10.1029/2004GL019412. ISSN 1944-8007.
- Gerber, R. Benny; Finlayson-Pitts, Barbara J.; Hammerich, Audrey Dell (2015-07-15). "Mechanism for formation of atmospheric Cl atom precursors in the reaction of dinitrogen oxides with HCl/Cl− on aqueous films" (PDF). Physical Chemistry Chemical Physics. 17 (29): 19360–19370. Bibcode:2015PCCP...1719360H. doi:10.1039/C5CP02664D. ISSN 1463-9084. PMID 26140681.
- Kelleher, Patrick J.; Menges, Fabian S.; DePalma, Joseph W.; Denton, Joanna K.; Johnson, Mark A.; Weddle, Gary H.; Hirshberg, Barak; Gerber, R. Benny (2017-09-18). "Trapping and Structural Characterization of the XNO2·NO3– (X = Cl, Br, I) Exit Channel Complexes in the Water-Mediated X– + N2O5 Reactions with Cryogenic Vibrational Spectroscopy". The Journal of Physical Chemistry Letters. 8 (19): 4710–4715. doi:10.1021/acs.jpclett.7b02120. ISSN 1948-7185. PMID 28898581.
