Cyclin-dependent kinase 6
Cell division protein kinase 6 (CDK6) is an enzyme encoded by the CDK6 gene.[5][6] It is regulated by cyclins, more specifically by Cyclin D proteins and Cyclin-dependent kinase inhibitor proteins.[7] The protein encoded by this gene is a member of the cyclin-dependent kinase, (CDK) family, which includes CDK4.[8] CDK family members are highly similar to the gene products of Saccharomyces cerevisiae cdc28, and Schizosaccharomyces pombe cdc2, and are known to be important regulators of cell cycle progression in the point of regulation named R or restriction point.[9]
This kinase is a catalytic subunit of the protein kinase complex, important for the G1 phase progression and G1/S transition of the cell cycle and the complex is composed also by an activating sub-unit; the cyclin D.[10] The activity of this kinase first appears in mid-G1 phase, which is controlled by the regulatory subunits including D-type cyclins and members of INK4 family of CDK inhibitors.[7] This kinase, as well as CDK4, has been shown to phosphorylate, and thus regulate the activity of, tumor suppressor Retinoblastoma protein making CDK6 an important protein in cancer development.[10]
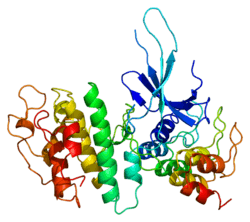
Structure
The CDK6 gene is conserved in eukaryotes, including the budding yeast and the nematode Caenorhabditis elegans.[11] The CDK6 gene is located on chromosome 7 in humans. The gene spans 231,706 base pairs and encodes a 326 amino acid protein with a kinase function.[6] The gene is overexpressed in cancers like lymphoma, leukemia, medulloblastoma and melanoma associated with chromosomal rearrangements.[6] The CDK6 protein contains a catalytic core composed of a serine/threonine domain.[12] This protein also contains an ATP-binding pocket, inhibitory and activating phosphorylation sites, a PSTAIRE-like cyclin-binding domain and an activating T-loop motif.[10] After binding the Cyclin in the PSTAIRE helix, the protein changes its conformational structure to expose the phosphorylation motif.[10] The protein can be found in the cytoplasm and the nucleus, however most of the active complexes are found in the nucleus of proliferating cells.[10]
Function
Cell cycle
In 1994, Matthew Meyerson and Ed Harlow investigated the product of a close analogous gene of CDK4.[7] This gene, identified as PLSTIRE was translated into a protein that interacted with the cyclins CD1, CD2 and CD3 (same as CDK4), but that was different from CDK4; the protein was then renamed CDK6 for simplicity.[7] In mammalian cells, cell cycle is activated by CDK6 in the early G1 phase[13] through interactions with cyclins D1, D2 and D3.[7] There are many changes in gene expression that are regulated through this enzyme.[14] After the complex is formed, the C-CDK6 enzymatic complex phosphorylates the protein pRb.[15] After its phosphorylation, pRb releases its binding partner E2F, a transcriptional activator, which in turn activates DNA replication.[16] The CDK6 complex ensures a point of switch to commit to division responding to external signals, like mitogens and growth factors.[17]
CDK6 is involved in a positive feedback loop that activates transcription factors through a reaction cascade.[18] Importantly, these C-CDK complexes act as a kinase, phosphorylating and inactivating the protein of Rb and p-Rb related “pocket proteins” p107 and p130.[19] While doing this, the CDK6 in conjunction with CDK4, act as a switch signal that first appears in G1,[7] directing the cell towards S phase of the cell cycle.[14]
CDK6 is important for the control of G1 to S phase transition.[7] However, in recent years, new evidence proved that the presence of CDK6 is not essential for proliferation in every cell type,[20] the cell cycle has a complex circuitry of regulation and the role of CDK6 might be more important in certain cell types than in others, where CDK4 or CDK2 can act as protein kinases compensating its role.[20][21]
Cellular development
In mutant Knockout mice of CDK6, the hematopoietic function is impaired, regardless of otherwise organism normal development.[20] This might hint additional roles of CDK6 in the development of blood components.[20] There are additional functions of CDK6 not associated with its kinase activity.[22] For example, CDK6 is involved in the differentiation of T cells, acting as an inhibitor of differentiation.[22] Even though CDK6 and CDK4 share 71% amino acid identity, this role in differentiation is unique to CDK6.[22] CDK6 has also been found to be important in the development of other cell lines, for example, CDK6 has a role in the alteration of the morphology of astrocytes[23] and in the development of other stem cells.[10][16]
DNA protection
CDK6 differs from CDK4 in other important roles.[24] For example, CDK6 plays a role in the accumulation of the apoptosis proteins p53 and p130, this accumulation keeps cells from entering cell division if there is DNA damage, activating pro- apoptotic pathways.[24]
Metabolic homeostasis
Studies in the metabolic control of cells have revealed yet another role of CDK6.[25] This new role is associated with the balance of the oxidative and non-oxidative branches of the pentose pathway in cells.[25] This pathway is a known route altered in cancer cells, when there is an aberrant overexpression of CDK6 and CDK4.[25] The overexpression of these proteins provides the cancer cells with a new hallmark capability of cancer; the deregulation of the cell metabolism.[25]
Centrosome stability
In 2013, researchers discovered yet another role of CDK6.[26] There is evidence that CDK6 associates with the centrosome and controls organized division and cell cycle phases in neuron production.[26] When the CDK6 gene is mutated in these developing lines, the centrosomes are not properly divided, this could lead to division problems such as aneuploidy, which in turns leads to health issues like primary microcephaly.[26]
Mechanisms of regulation
CDK6 is positively regulated primarily by its union to the D cyclins D1, D2 and D3. If this subunit of the complex is not available, CDK6 is not active or available to phosphorylate the pRb substrate.[9] An additional positive activator needed by CDK6 is the phosphorylation in a conserved threonine residue located in 177 position, this phosphorylation is done by the cdk-activating kinases, CAK.[27] Additionally, CDK6 can be phosphorylated and activated by the Kaposi's sarcoma-associated herpes virus, stimulating the CDK6 over activation and uncontrolled cell proliferation.[28]
CDK6 is negatively regulated by binding to certain inhibitors that can be classified in two groups;[29] CKIs or CIP/KIP family members like the protein p21[16] and p27 act blocking and inhibiting the assembled C-CDKs binding complex enzymes[27] in their catalytic domain.[30]
Furthermore, inhibitors of the INK4 family members like p15, p16, p18 and p19 inhibit the monomer of CDK6, preventing the complex formation.[19][31]
Clinical relevance
CDK6 is a protein kinase activating cell proliferation, it is involved in an important point of restriction in the cell cycle.[18] For this reason, CDK6 and other regulators of the G1 phase of the cell cycle are known to be unbalanced in more than 80-90% of tumors.[9] In cervical cancer cells, CDK6 function has been shown to be altered indirectly by the p16 inhibitor.[31] CDK6 is also overexpressed in tumors that exhibit drug resistance, for example glioma malignancies exhibit resistance to chemotherapy using temozolomide (TMZ) when they have a mutation overexpressing CDK6.[32] Likewise, the overexpression of CDK6 is also associated with resistance to hormone therapy using the anti oestrogen Fluvestrant in breast cancer.[33]
Cancer
Loss of normal cell cycle control is the first step to developing different hallmarks of cancer; alterations of CDK6 can directly or indirectly affect the following hallmarks; disregulated cell cellular energetics, sustaining of proliferative signaling, evading growth suppressors and inducing angiogenesis,[9] for example, deregulation of CDK6 has been shown to be important in lymphoid malignancies by increasing angiogenesis, a hallmark of cancer.[19] These features are reached through upregulation of CDK6 due to chromosome alterations or epigenetic dysregulations.[9] Additionally, CDK6 might be altered through genomic instability, a mechanism of downregulation of tumor suppressor genes; this represents another evolving hallmark of cancer.[34]
Medulloblastoma
Medulloblastoma is the most common cause of brain cancer in children.[35] About a third of these cancers have upregulated CDK6, representing a marker for poor prognosis for this disease.[35] Since it is so common for these cells to have alterations in CDK6, researchers are seeking for ways to downregulate CDK6 expression acting specifically in those cell lines. The MicroRNA (miR) -124 has successfully controlled cancer progression in an in-vitro setting for medulloblastoma and glioblastoma cells.[35] Furthermore, researchers have found that it successfully reduces the growth of xenograft tumors in rat models.[35]
As a drug target
The direct targeting of CDK6 and CDK4 should be used with caution in the treatment of cancer, because these enzymes are important for the cell cycle of normal cells as well.[35] Furthermore, small molecules targeting these proteins might increase drug resistance events.[35] However, these kinases have been shown to be useful as coadjuvants in breast cancer chemotherapy.[36] Another indirect mechanism for the control of CDK6 expression, is the use of a mutated D-cyclin that binds with high affinity to CDK6, but does not induce its kinase activity.[36] this mechanism was studied in the development of mammary tumorigenesis in rat cells, however, the clinical effects have not yet been shown in human patients.[36] A
Interactions
Cyclin-dependent kinase 6 interacts with:
See also
References
- GRCh38: Ensembl release 89: ENSG00000105810 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000040274 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH (Aug 1992). "A family of human cdc2-related protein kinases". The EMBO Journal. 11 (8): 2909–17. doi:10.1002/j.1460-2075.1992.tb05360.x. PMC 556772. PMID 1639063.
- "Entrez Gene: CDK6 cyclin-dependent kinase 6".
- Meyerson, M; Harlow, E (1994). "Identification of G1 Kinase Activity for cdk6, a Novel Cyclin D Partner". Molecular and Cellular Biology. 14 (3): 2077–86. doi:10.1128/MCB.14.3.2077. PMC 358568. PMID 8114739.
- Robbins Basic Pathology by Vinay Kumar, Abul K. Abbas, and Jon C. Aster | eBook on, accessed April 21, 2014, https://www.inkling.com/store/book/robbins-basic-pathology-kumar-abbas-aster-9th/?chapterId=d0de80fcb2d4401c91c3045fcf0f45e1.
- Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M (May 2013). "Targeting cell cycle regulation in cancer therapy". Pharmacology & Therapeutics. 138 (2): 255–71. doi:10.1016/j.pharmthera.2013.01.011. PMID 23356980.
- Lim S, Kaldis P (Aug 2013). "Cdks, cyclins and CKIs: roles beyond cell cycle regulation". Development. 140 (15): 3079–93. doi:10.1242/dev.091744. PMID 23861057.
- Liu, Ji; Kipreos, Edward T. (2000). "Evolution of Cyclin-Dependent Kinases (CDKs) and CDK-Activating Kinases (CAKs): Differential Conservation of CAKs in Yeast and Metazoa". Molecular Biology and Evolution. 17 (7): 1061–74. doi:10.1093/oxfordjournals.molbev.a026387. PMID 10889219.
- Reinhardt HC, Yaffe MB (Sep 2013). "Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response". Nature Reviews Molecular Cell Biology. 14 (9): 563–80. doi:10.1038/nrm3640. PMID 23969844.
- Harvey Lodish et al., Molecular Cell Biology. 4th Edition., 2000, https://www.ncbi.nlm.nih.gov/books/NBK21497/.
- Bertoli C, Skotheim JM, de Bruin RA (Aug 2013). "Control of cell cycle transcription during G1 and S phases". Nature Reviews Molecular Cell Biology. 14 (8): 518–28. doi:10.1038/nrm3629. PMC 4569015. PMID 23877564.
- Ezhevsky SA, Ho A, Becker-Hapak M, Davis PK, Dowdy SF (Jul 2001). "Differential regulation of retinoblastoma tumor suppressor protein by G(1) cyclin-dependent kinase complexes in vivo". Molecular and Cellular Biology. 21 (14): 4773–84. doi:10.1128/MCB.21.14.4773-4784.2001. PMC 87164. PMID 11416152.
- Grossel MJ, Hinds PW (Feb 2006). "Beyond the cell cycle: a new role for Cdk6 in differentiation". Journal of Cellular Biochemistry. 97 (3): 485–93. doi:10.1002/jcb.20712. PMID 16294322.
- Bartek, J; Lukas, J (2001). "Mammalian G1- and S-Phase Checkpoints in Response to DNA Damage". Current Opinion in Cell Biology. 13 (6): 738–47. doi:10.1016/s0955-0674(00)00280-5. PMID 11698191.
- Aarts M, Linardopoulos S, Turner NC (Aug 2013). "Tumour selective targeting of cell cycle kinases for cancer treatment". Current Opinion in Pharmacology. 13 (4): 529–35. doi:10.1016/j.coph.2013.03.012. PMID 23597425.
- Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, Schäfer M, Fajmann S, Schlederer M, Schiefer AI, Reichart U, Mayerhofer M, Hoeller C, Zöchbauer-Müller S, Kerjaschki D, Bock C, Kenner L, Hoefler G, Freissmuth M, Green AR, Moriggl R, Busslinger M, Malumbres M, Sexl V (Aug 2013). "A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis". Cancer Cell. 24 (2): 167–81. doi:10.1016/j.ccr.2013.07.012. PMC 3743049. PMID 23948297.
- Katarzyna Kozar and Piotr Sicinski, "Cell Cycle Progression without Cyclin D-CDK4 and Cyclin D-CDK6 Complexes," Cell Cycle 4, no. 3 (March 2005): 388–91
- Malumbres M, Sotillo R, Santamaría D, Galán J, Cerezo A, Ortega S, Dubus P, Barbacid M (Aug 2004). "Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6". Cell. 118 (4): 493–504. doi:10.1016/j.cell.2004.08.002. PMID 15315761.
- Martha J Grossel and Philip W Hinds, "From Cell Cycle to Differentiation: An Expanding Role for cdk6," Cell Cycle 5, no. 3 (February 2006): 266–70
- Ericson, Karen K.; et al. (2003). "Expression of Cyclin-Dependent Kinase 6, but Not Cyclin-Dependent Kinase 4, Alters Morphology of Cultured Mouse Astrocytes11NSF under CAREER Grant #9984454 to Martha J. Grossel". Molecular Cancer Research. 1 (9): 654–64.
- Nagasawa M, Gelfand EW, Lucas JJ (May 2001). "Accumulation of high levels of the p53 and p130 growth-suppressing proteins in cell lines stably over-expressing cyclin-dependent kinase 6 (cdk6)". Oncogene. 20 (23): 2889–99. doi:10.1038/sj.onc.1204396. PMID 11420701.
- Zanuy M, Ramos-Montoya A, Villacañas O, Canela N, Miranda A, Aguilar E, Agell N, Bachs O, Rubio-Martinez J, Pujol MD, Lee WN, Marin S, Cascante M (Jun 2012). "Cyclin-dependent kinases 4 and 6 control tumor progression and direct glucose oxidation in the pentose cycle". Metabolomics. 8 (3): 454–64. doi:10.1007/s11306-011-0328-x. PMC 3361763. PMID 22661920.
- Hussain, Muhammad S; et al. (2013). "CDK6 Associates with the Centrosome during Mitosis and Is Mutated in a Large Pakistani Family with Primary Microcephaly". Human Molecular Genetics. 22 (25): 5199–5214. doi:10.1093/hmg/ddt374. PMID 23918663.
- LaBaer, J; et al. (1997). "New Functional Activities for the p21 Family of CDK Inhibitors". Genes & Development. 11 (7): 847–62. doi:10.1101/gad.11.7.847. PMID 9106657.
- Kaldis P (Mar 2005). "The N-terminal peptide of the Kaposi's sarcoma-associated herpesvirus (KSHV)-cyclin determines substrate specificity". The Journal of Biological Chemistry. 280 (12): 11165–74. doi:10.1074/jbc.M408887200. PMID 15664993.
- Nurse, P (2000). "A Long Twentieth Century of the Cell Cycle and beyond". Cell. 100 (1): 71–78. doi:10.1016/s0092-8674(00)81684-0. PMID 10647932.
- Bockstaele L, Kooken H, Libert F, Paternot S, Dumont JE, de Launoit Y, Roger PP, Coulonval K (Jul 2006). "Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4(CDK4): its relationship with cyclins and CDK "inhibitors"". Molecular and Cellular Biology. 26 (13): 5070–85. doi:10.1128/MCB.02006-05. PMC 1489149. PMID 16782892.
- Khleif, S N; et al. (1996). "Inhibition of Cyclin D-CDK4/CDK6 Activity Is Associated with an E2F-Mediated Induction of Cyclin Kinase Inhibitor Activity". Proceedings of the National Academy of Sciences of the United States of America. 93 (9): 4350–54. doi:10.1073/pnas.93.9.4350. PMC 39540. PMID 8633069.
- Li B, He H, Tao BB, Zhao ZY, Hu GH, Luo C, Chen JX, Ding XH, Sheng P, Dong Y, Zhang L, Lu YC (Sep 2012). "Knockdown of CDK6 enhances glioma sensitivity to chemotherapy". Oncology Reports. 28 (3): 909–14. doi:10.3892/or.2012.1884. PMID 22736304.
- Giessrigl B, Schmidt WM, Kalipciyan M, Jeitler M, Bilban M, Gollinger M, Krieger S, Jäger W, Mader RM, Krupitza G (Nov 2013). "Fulvestrant induces resistance by modulating GPER and CDK6 expression: implication of methyltransferases, deacetylases and the hSWI/SNF chromatin remodelling complex". British Journal of Cancer. 109 (10): 2751–62. doi:10.1038/bjc.2013.583. PMC 3833203. PMID 24169358.
- Negrini S, Gorgoulis VG, Halazonetis TD (Mar 2010). "Genomic instability--an evolving hallmark of cancer". Nature Reviews Molecular Cell Biology. 11 (3): 220–28. doi:10.1038/nrm2858. PMID 20177397.
- Silber J, Hashizume R, Felix T, Hariono S, Yu M, Berger MS, Huse JT, VandenBerg SR, James CD, Hodgson JG, Gupta N (Jan 2013). "Expression of miR-124 inhibits growth of medulloblastoma cells". Neuro-Oncology. 15 (1): 83–90. doi:10.1093/neuonc/nos281. PMC 3534424. PMID 23172372.
- Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW (Jan 2006). "Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis". Cancer Cell. 9 (1): 13–22. doi:10.1016/j.ccr.2005.12.019. PMID 16413468.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3: 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O'Keefe CL, Matera AG, Xiong Y (Dec 1994). "Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function". Genes & Development. 8 (24): 2939–52. doi:10.1101/gad.8.24.2939. PMID 8001816.
- Jeffrey PD, Tong L, Pavletich NP (Dec 2000). "Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors". Genes & Development. 14 (24): 3115–25. doi:10.1101/gad.851100. PMC 317144. PMID 11124804.
- Lin J, Jinno S, Okayama H (Apr 2001). "Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell's proliferation competence". Oncogene. 20 (16): 2000–9. doi:10.1038/sj.onc.1204375. PMID 11360184.
- Sugimoto M, Nakamura T, Ohtani N, Hampson L, Hampson IN, Shimamoto A, Furuichi Y, Okumura K, Niwa S, Taya Y, Hara E (Nov 1999). "Regulation of CDK4 activity by a novel CDK4-binding protein, p34(SEI-1)". Genes & Development. 13 (22): 3027–33. doi:10.1101/gad.13.22.3027. PMC 317153. PMID 10580009.
- Meyerson M, Harlow E (Mar 1994). "Identification of G1 kinase activity for cdk6, a novel cyclin D partner". Molecular and Cellular Biology. 14 (3): 2077–86. doi:10.1128/MCB.14.3.2077. PMC 358568. PMID 8114739.
- Fåhraeus R, Paramio JM, Ball KL, Laín S, Lane DP (Jan 1996). "Inhibition of pRb phosphorylation and cell-cycle progression by a 20-residue peptide derived from p16CDKN2/INK4A". Current Biology. 6 (1): 84–91. doi:10.1016/s0960-9822(02)00425-6. PMID 8805225.
- Russo AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP (Sep 1998). "Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a". Nature. 395 (6699): 237–43. doi:10.1038/26155. PMID 9751050.
- Kaldis P, Ojala PM, Tong L, Mäkelä TP, Solomon MJ (Dec 2001). "CAK-independent activation of CDK6 by a viral cyclin". Molecular Biology of the Cell. 12 (12): 3987–99. doi:10.1091/mbc.12.12.3987. PMC 60770. PMID 11739795.
- Cheng A, Kaldis P, Solomon MJ (Nov 2000). "Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms". The Journal of Biological Chemistry. 275 (44): 34744–9. doi:10.1074/jbc.M006210200. PMID 10934208.
Further reading
- Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O (Sep 1995). "Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence" (PDF). Nature. 377 (6547 Suppl): 3–174. PMID 7566098.
- Aprelikova O, Xiong Y, Liu ET (Aug 1995). "Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase". The Journal of Biological Chemistry. 270 (31): 18195–7. doi:10.1074/jbc.270.31.18195. PMID 7629134.
- Lucas JJ, Szepesi A, Modiano JF, Domenico J, Gelfand EW (Jun 1995). "Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin D-associated cdk4 homologue in normal human T lymphocytes". Journal of Immunology. 154 (12): 6275–84. PMID 7759865.
- Bullrich F, MacLachlan TK, Sang N, Druck T, Veronese ML, Allen SL, Chiorazzi N, Koff A, Heubner K, Croce CM (Mar 1995). "Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27Kip1, to regions involved in human cancer". Cancer Research. 55 (6): 1199–205. PMID 7882308.
- Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O'Keefe CL, Matera AG, Xiong Y (Dec 1994). "Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function". Genes & Development. 8 (24): 2939–52. doi:10.1101/gad.8.24.2939. PMID 8001816.
- Meyerson M, Harlow E (Mar 1994). "Identification of G1 kinase activity for cdk6, a novel cyclin D partner". Molecular and Cellular Biology. 14 (3): 2077–86. doi:10.1128/MCB.14.3.2077. PMC 358568. PMID 8114739.
- Fåhraeus R, Paramio JM, Ball KL, Laín S, Lane DP (Jan 1996). "Inhibition of pRb phosphorylation and cell-cycle progression by a 20-residue peptide derived from p16CDKN2/INK4A". Current Biology. 6 (1): 84–91. doi:10.1016/S0960-9822(02)00425-6. PMID 8805225.
- Bonaldo MF, Lennon G, Soares MB (Sep 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta GF, Gyuris J (Apr 1997). "Interaction between Cdc37 and Cdk4 in human cells". Oncogene. 14 (16): 1999–2004. doi:10.1038/sj.onc.1201036. PMID 9150368.
- Nagasawa M, Melamed I, Kupfer A, Gelfand EW, Lucas JJ (Jun 1997). "Rapid nuclear translocation and increased activity of cyclin-dependent kinase 6 after T cell activation". Journal of Immunology. 158 (11): 5146–54. PMID 9164930.
- Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF (Sep 1997). "Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb". Proceedings of the National Academy of Sciences of the United States of America. 94 (20): 10699–704. doi:10.1073/pnas.94.20.10699. PMC 23451. PMID 9380698.
- Fåhraeus R, Laín S, Ball KL, Lane DP (Feb 1998). "Characterization of the cyclin-dependent kinase inhibitory domain of the INK4 family as a model for a synthetic tumour suppressor molecule". Oncogene. 16 (5): 587–96. doi:10.1038/sj.onc.1201580. PMID 9482104.
- Gonzales AJ, Goldsworthy TL, Fox TR (Jun 1998). "Chemical transformation of mouse liver cells results in altered cyclin D-CDK protein complexes". Carcinogenesis. 19 (6): 1093–102. doi:10.1093/carcin/19.6.1093. PMID 9667749.
- Russo AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP (Sep 1998). "Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a". Nature. 395 (6699): 237–43. doi:10.1038/26155. PMID 9751050.
- Brotherton DH, Dhanaraj V, Wick S, Brizuela L, Domaille PJ, Volyanik E, Xu X, Parisini E, Smith BO, Archer SJ, Serrano M, Brenner SL, Blundell TL, Laue ED (Sep 1998). "Crystal structure of the complex of the cyclin D-dependent kinase Cdk6 bound to the cell-cycle inhibitor p19INK4d". Nature. 395 (6699): 244–50. doi:10.1038/26164. PMID 9751051.
- Jiang W, Wells NJ, Hunter T (May 1999). "Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6". Proceedings of the National Academy of Sciences of the United States of America. 96 (11): 6193–8. doi:10.1073/pnas.96.11.6193. PMC 26858. PMID 10339564.
- Yarbrough WG, Buckmire RA, Bessho M, Liu ET (Sep 1999). "Biologic and biochemical analyses of p16(INK4a) mutations from primary tumors". Journal of the National Cancer Institute. 91 (18): 1569–74. doi:10.1093/jnci/91.18.1569. PMID 10491434.
- Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC (Sep 1999). "Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1". Cell. 98 (6): 859–69. doi:10.1016/S0092-8674(00)81519-6. PMID 10499802.
- Grossel MJ, Baker GL, Hinds PW (Oct 1999). "cdk6 can shorten G(1) phase dependent upon the N-terminal INK4 interaction domain". The Journal of Biological Chemistry. 274 (42): 29960–7. doi:10.1074/jbc.274.42.29960. PMID 10514479.
External links
- Cyclin-Dependent+Kinase+6 at the US National Library of Medicine Medical Subject Headings (MeSH)
- CDK6 human gene location in the UCSC Genome Browser.
- CDK6 human gene details in the UCSC Genome Browser.
- Genecards
- UniProt