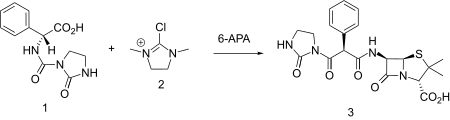
Azlocillin
Azlocillin is an acylampicillin antibiotic with an extended spectrum of activity and greater in vitro potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including Pseudomonas aeruginosa and, in contrast to most cephalosporins, exhibits activity against enterococci. Azlocillin has been identified as a potential candidate against Lyme disease.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.483 |
| Chemical and physical data | |
| Formula | C20H23N5O6S |
| Molar mass | 461.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Spectrum of bacterial susceptibility
Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms.[2]
- Escherichia coli 1 μg/mL – 32 μg/mL
- Haemophilus spp. 0.03 μg/mL – 2 μg/mL
- Pseudomonas aeruginosa 4 μg/mL – 6.25 μg/mL
Synthesis
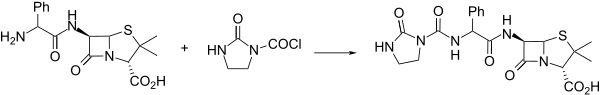
An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chloride (2) and then condensation with 6-APA.
See also
References
- https://scopeblog.stanford.edu/2020/03/30/lyme-disease-bacteria-eradicated-by-new-drug-in-early-tests/
- "Azlocillin sodium salt Susceptibility and Minimum Inhibitory and Concentration (MIC) Data" (PDF). The Antimicrobial Index. toku-e.com.
- Koenig HB, Metzer KG, Offe HA, Schroeck W (1982). "Azlocillin. Ein Neues Penicillin aus der Acylureidoreihe: Synthese und Chemische Eigenschaften". Eur. J. Med. Chem. - Chim. Ther. (in German). 17 (1): 59–63.
- Bauer VJ, Safir SR (November 1966). "Octamethylbiguanide perchlorate". Journal of Medicinal Chemistry. 9 (6): 980–1. doi:10.1021/jm00324a056. PMID 4291383.