Phenylglycine
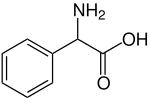
Phenylglycine is the organic compound with the formula C6H5CH(NH2)CO2H. It is a non-proteinogenic alpha amino acid related to alanine, but with a phenyl group in place of the methyl group. It is a white solid. The compound exhibits some biological activity.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-2-phenylacetic acid | |
| Other names
2-Phenylglycine; Aminophenylacetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 608018 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.735 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H9NO2 | |
| Molar mass | 151.165 g·mol−1 |
| Appearance | White solid |
| Melting point | 290 °C (554 °F; 563 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It is prepared from benzaldehyde by amino cyanation (Strecker synthesis).[2] It can also be prepared from glyoxal[3] and by reductive amination of phenylglyoxylic acid.
Ester
The ester methyl α‐phenylglycinate is used to convert carboxylic acids into homologated unsaturated ketones. These reactions proceed via cyclization of phenylglycinamides to oxazolones, which can be reductively cleaved with chromous reagents.[4]
gollark: You could install an FTP server and something something FUSE-mount that.
gollark: Anyway, I simply have a vast mess of nested folders with names like `data`, which are synchronized between my server, laptop and sometimes phone.
gollark: > rounded corners
gollark: Well, I mostly use syncthing for interaction with my phone.
gollark: Oh, `jmtpfs` is the FUSE thing and you dislike it?
See also
References
- Watkins, Jeff; Collingridge, Graham (1994). "Phenylglycine derivatives as antagonists of metabotropic glutamate receptors". Trends in Pharmacological Sciences. 15 (9): 333–42. doi:10.1016/0165-6147(94)90028-0. PMID 7992387.CS1 maint: uses authors parameter (link)
- Robert E. Steiger (1942). "dl-α-Aminophenylacetic Acid". Org. Synth. 22: 23. doi:10.15227/orgsyn.022.0023.
- Agami, Claude; Couty, Francois; Puchot-Kadouri, Cathy (1998). "Asymmetric synthesis of α-amino acids from a chiral masked form of glyoxal". Synlett. 1998 (5): 449–456. doi:10.1055/s-1998-1685.CS1 maint: uses authors parameter (link)
- Wolfgang Steglich Stefan Jaroch (2001). "Methyl α-Phenylglycinate". Methyl α‐Phenylglycinate. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm229. ISBN 0471936235.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.