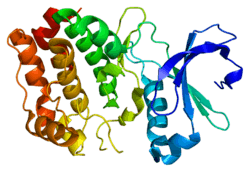
Aurora A kinase
Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.[5][6]
Aurora A is a member of a family of mitotic serine/threonine kinases. It is implicated with important processes during mitosis and meiosis whose proper function is integral for healthy cell proliferation. Aurora A is activated by one or more phosphorylations[7] and its activity peaks during the G2 phase to M phase transition in the cell cycle.[8]
Discovery
The aurora kinases were first identified in 1990 during a cDNA screen of Xenopus eggs.[7] The kinase discovered, Eg2, is now referred to as Aurora A.[9] It was not until 1998, however, that Aurora A's meiotic and mitotic importance was realized.[7]
Aurora kinase family
The human genome contains three members the Aurora kinase family: Aurora A kinase, Aurora B kinase and Aurora C kinase. The Xenopus, Drosophila, and Caenorhabditis elegans genomes, on the other hand, contain orthologues only to Aurora A and Aurora B.[7]
In all studied species, the three Aurora mitotic kinases localize to the centrosome[9] during different phases of mitosis.[7] The family members have highly conserved C-terminal catalytic domains. Their N-terminal domains, however, exhibit a large degree of variance in the size and sequence.[9]
Aurora A and Aurora B kinases play important roles in mitosis. The Aurora A kinase is associated with centrosome maturation and separation and thereby regulates spindle assembly and stability. The Aurora B kinase is a chromosome passenger protein and regulates chromosome segregation and cytokinesis.
Although there is evidence to suggest that Aurora C might be a chromosomal passenger protein, the cellular function of it is less clear.
Localization
Aurora A localizes next to the centrosome late in the G1 phase and early in the S phase. As the cell cycle progresses, concentrations of Aurora A increase and the kinase associates with the mitotic poles and the adjacent spindle microtubules. Aurora A remains associated with the spindles through telophase.[7] Right before mitotic exit, Aurora A relocalizes to the mid-zone of the spindle.[10]
Mitosis
During mitosis, a mitotic spindle is assembled by using microtubules to tether together the mother centrosome to its daughter. The resulting mitotic spindle is then used to propel apart the sister chromosomes into what will become the two new daughter cells. Aurora A is critical for proper formation of mitotic spindle. It is required for the recruitment of several different proteins important to the spindle formation. Among these target proteins are TACC, a microtubule-associated protein that stabilizes centrosomal microtubules and Kinesin 5, a motor protein involved in the formation of the bipolar mitotic spindle.[7] γ-tubulins, the base structure from which centrosomal microtubules polymerize, are also recruited by Aurora A. Without Aurora A the centrosome does not accumulate the quantity of γ-tubulin that normal centrosomes recruit prior to entering anaphase. Though the cell cycle continues even in the absence of deficient γ-tubulin, the centrosome never fully matures; it organizes fewer aster microtubules than normal.[8]
Furthermore, Aurora A is necessary for the proper separation of the centrosomes after the mitotic spindle has been formed. Without Aurora A, the mitotic spindle, depending on the organism, will either never separate or will begin to separate only to collapse back onto itself.[8] In the case of the former, it has been suggested that Aurora A cooperates with the kinase Nek2 in Xenopus to dissolve the structure tethering the cell's centrosomes together. Therefore, without proper expression of Aurora A, the cell's centrosomes are never able to separate.[10]
Aurora A also assures proper organization and alignment of the chromosomes during prometaphase. It is directly involved in the interaction of the kinetochore, the part of the chromosome at which the mitotic spindle attaches and pulls, and the mitotic spindle's extended microtubules. It is speculated that Aurora B cooperates with Aurora A to complete this task. In the absence of Aurora A mad2, a protein that normally dissipates once a proper kinetochore-microtubule connection is made, remains present even into metaphase.[10]
Finally, Aurora A helps orchestrate an exit from mitosis by contributing to the completion of cytokinesis- the process by which the cytoplasm of the parent cell is split into two daughter cells. During citokinesis the mother centriole returns to the mid-body of the mitotic cell at the end of mitosis and causes the central microtubules to release from the mid-body. The release allows mitosis to run to completion. Though the exact mechanism by which Aurora A aids cytokinesis is unknown, it is well documented that it relocalizes to the mid-body immediately before the completion of mitosis.[10]
Intriguingly, abolishment of Aurora A through RNAi interference results in different mutant phenotypes in different organisms and cell types.[10] For example, deletion of Aurora A in C. elegans results in an initial separation of the cell's centrosomes followed by an immediate collapse of the asters. In Xenopus, deletion disallows the mitotic spindle from ever even forming.[8] And in Drosophila, flies without Aurora A will effectively form spindles and separate but the aster microtubules will be dwarfed. These observations suggests that while Aurora-A has orthologues in many different organisms, it may play a similar but slightly different role in each.[10]
Meiosis
Aurora A phosphorylation directs the cytoplasmic polyadenylation translation of mRNA's, like the MAP kinase kinase kinase protein MOS, that are vital to the completion of meiosis in Xenopus Oocytes.[9] Prior to the first meiotic metaphase, Aurora A induces the synthesis of MOS. The MOS protein accumulates until it exceeds a threshold and then transduces the phosphorylation cascade in the map kinase pathway. This signal subsequently activates the kinase RSK which in turn binds to the protein Myt1. Myt1, in complex with RSK, is now unable to inhibit cdc2. As a consequence, cdc2 permits entry into meiosis.[7] A similar Aurora A dependent process regulates the transition from meiosis I-meiosis II.
Furthermore, Aurora A has been observed to have a biphasic pattern of activation during progression through meiosis. It has been suggested that the fluctuations, or phases, of Aurora A activation are dependent on a positive-feedback mechanism with a p13SUC1-associated protein kinase[10]
Protein translation
Aurora A is not only implicated with the translation of MOS during meiosis but also in the polyadenylation and subsequent translation of neural mRNAs whose protein products are associated with synaptic plasticity.[10]
Clinical significance
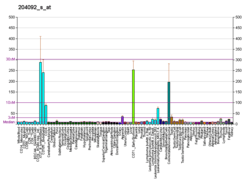
Aurora A dysregulation has been associated with high occurrence of cancer. For example, one study showed over-expression of Aurora A in 94 percent of the invasive tissue growth in breast cancer, while surrounding, healthy tissues had normal levels of Aurora A expression.[7] Aurora A has also been shown to be involved in the Epithelial–mesenchymal transition and Neuroendocrine Transdifferentiation of Prostate Cancer cells in aggressive disease.[11]
Dysregulation of Aurora A may lead to cancer because Aurora A is required for the completion of cytokinesis. If the cell begins mitosis, duplicates its DNA, but is then not able to divide into two separate cells it becomes an aneuploid- containing more chromosomes than normal. Aneuploidy is a trait of many cancerous tumors.[10] Ordinarily, Aurora A expression levels are kept in check by the tumor suppressor protein p53.[7]
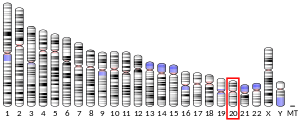
Mutations of the chromosome region that contains Aurora A, 20q13, are generally considered to have a poor prognosis.[7]
Osimertinib and rociletinib, two anti cancer drugs for lung cancer, work by shutting off mutant EGFR, which initially kills cancerous tumors, but the tumors rewire and activate Aurora kinase A, becoming cancerous growths again. According to a 2018 study, targeting both EGFR and Aurora prevents return of drug resistant tumors.[12]
Interactions
Aurora A kinase has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000087586 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027496 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Sen S, Zhou H, White RA (May 1997). "A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines". Oncogene. 14 (18): 2195–200. doi:10.1038/sj.onc.1201065. PMID 9174055.
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S (October 1998). "Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation". Nat. Genet. 20 (2): 189–93. doi:10.1038/2496. PMID 9771714.
- Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV (2004). "Aurora A, meiosis and mitosis" (PDF). Biol. Cell. 96 (3): 215–29. doi:10.1016/j.biolcel.2003.09.008. PMID 15182704. Archived from the original (PDF) on 2007-02-05. Retrieved 2007-03-05.
- Hannak E, Kirkham M, Hyman AA, Oegema K (December 2001). "Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans". J. Cell Biol. 155 (7): 1109–16. doi:10.1083/jcb.200108051. PMC 2199344. PMID 11748251.
- Ma C, Cummings C, Liu XJ (March 2003). "Biphasic activation of Aurora-A kinase during the meiosis I- meiosis II transition in Xenopus oocytes". Mol. Cell. Biol. 23 (5): 1703–16. doi:10.1128/MCB.23.5.1703-1716.2003. PMC 151708. PMID 12588989.
- Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H (December 2003). "Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells". J. Biol. Chem. 278 (51): 51786–95. doi:10.1074/jbc.M306275200. PMID 14523000.
- Nouri M, Ratther E, Stylianou N, Nelson CC, Hollier BG, Williams ED (2014). "Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention". Front Oncol. 4: 370. doi:10.3389/fonc.2014.00370. PMC 4274903. PMID 25566507.
- https://medicalxpress.com/news/2018-11-cancer-achilles-heel-drug-resistant-tumors.html
- Sakai H, Urano T, Ookata K, Kim MH, Hirai Y, Saito M, Nojima Y, Ishikawa F (December 2002). "MBD3 and HDAC1, two components of the NuRD complex, are localized at Aurora-A-positive centrosomes in M phase". J. Biol. Chem. 277 (50): 48714–23. doi:10.1074/jbc.M208461200. PMID 12354758.
- Du J, Hannon GJ (December 2002). "The centrosomal kinase Aurora-A/STK15 interacts with a putative tumor suppressor NM23-H1". Nucleic Acids Res. 30 (24): 5465–75. doi:10.1093/nar/gkf678. PMC 140054. PMID 12490715.
- Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS (September 2002). "Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function". EMBO J. 21 (17): 4491–9. doi:10.1093/emboj/cdf409. PMC 126178. PMID 12198151.
- Delaval B, Ferrand A, Conte N, Larroque C, Hernandez-Verdun D, Prigent C, Birnbaum D (June 2004). "Aurora B -TACC1 protein complex in cytokinesis". Oncogene. 23 (26): 4516–22. doi:10.1038/sj.onc.1207593. PMID 15064709.
- Conte N, Delaval B, Ginestier C, Ferrand A, Isnardon D, Larroque C, Prigent C, Séraphin B, Jacquemier J, Birnbaum D (November 2003). "TACC1-chTOG-Aurora A protein complex in breast cancer". Oncogene. 22 (50): 8102–16. doi:10.1038/sj.onc.1206972. PMID 14603251.
- Kufer TA, Silljé HH, Körner R, Gruss OJ, Meraldi P, Nigg EA (August 2002). "Human TPX2 is required for targeting Aurora-A kinase to the spindle". J. Cell Biol. 158 (4): 617–23. doi:10.1083/jcb.200204155. PMC 2174010. PMID 12177045.
- Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BA, Nagase H, Burn J, Ball S, Almeida M, Linardopoulos S, Balmain A (August 2003). "Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human". Nat. Genet. 34 (4): 403–12. doi:10.1038/ng1220. PMID 12881723.
Further reading
- Ferchichi I, Stambouli N, Marrackchi R, Arlot Y, Prigent C, Fadiel A, Odunsi K, Ben Ammar Elgaaied A, Hamza A (January 2010). "Experimental and computational studies indicate specific binding of pVHL protein to Aurora-A kinase". J Phys Chem B. 114 (3): 1486–97. doi:10.1021/jp909869g. PMID 20047310.
- Nigg EA (2001). "Mitotic kinases as regulators of cell division and its checkpoints". Nat. Rev. Mol. Cell Biol. 2 (1): 21–32. doi:10.1038/35048096. PMID 11413462.
- Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, Okano Y (1997). "Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1". J. Biol. Chem. 272 (21): 13766–71. doi:10.1074/jbc.272.21.13766. PMID 9153231.
- Shindo M, Nakano H, Kuroyanagi H, Shirasawa T, Mihara M, Gilbert DJ, Jenkins NA, Copeland NG, Yagita H, Okumura K (1998). "cDNA cloning, expression, subcellular localization, and chromosomal assignment of mammalian aurora homologues, aurora-related kinase (ARK) 1 and 2". Biochem. Biophys. Res. Commun. 244 (1): 285–92. doi:10.1006/bbrc.1998.8250. PMID 9514916.
- Kimura M, Matsuda Y, Eki T, Yoshioka T, Okumura K, Hanaoka F, Okano Y (1997). "Assignment of STK6 to human chromosome 20q13.2-->q13.3 and a pseudogene STK6P to 1q41-->q42". Cytogenet. Cell Genet. 79 (3–4): 201–3. doi:10.1159/000134721. PMID 9605851.
- Farruggio DC, Townsley FM, Ruderman JV (1999). "Cdc20 associates with the kinase aurora2/Aik". Proc. Natl. Acad. Sci. U.S.A. 96 (13): 7306–11. doi:10.1073/pnas.96.13.7306. PMC 22081. PMID 10377410.
- Walter AO, Seghezzi W, Korver W, Sheung J, Lees E (2000). "The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation". Oncogene. 19 (42): 4906–16. doi:10.1038/sj.onc.1203847. PMID 11039908.
- Hartley JL, Temple GF, Brasch MA (2000). "DNA cloning using in vitro site-specific recombination". Genome Res. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Simpson JC, Wellenreuther R, Poustka A, Pepperkok R, Wiemann S (2000). "Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing". EMBO Rep. 1 (3): 287–92. doi:10.1093/embo-reports/kvd058. PMC 1083732. PMID 11256614.
- Katayama H, Zhou H, Li Q, Tatsuka M, Sen S (2001). "Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle". J. Biol. Chem. 276 (49): 46219–24. doi:10.1074/jbc.M107540200. PMID 11551964.
- Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P (2002). "Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases". Mol. Cell. Biol. 22 (3): 874–85. doi:10.1128/MCB.22.3.874-885.2002. PMC 133550. PMID 11784863.
- Tanaka M, Ueda A, Kanamori H, Ideguchi H, Yang J, Kitajima S, Ishigatsubo Y (2002). "Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1". J. Biol. Chem. 277 (12): 10719–26. doi:10.1074/jbc.M108252200. PMID 11790771.
- Meraldi P, Honda R, Nigg EA (2002). "Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells". EMBO J. 21 (4): 483–92. doi:10.1093/emboj/21.4.483. PMC 125866. PMID 11847097.
- Lauffart B, Howell SJ, Tasch JE, Cowell JK, Still IH (2002). "Interaction of the transforming acidic coiled-coil 1 (TACC1) protein with ch-TOG and GAS41/NuBI1 suggests multiple TACC1-containing protein complexes in human cells". Biochem. J. 363 (Pt 1): 195–200. doi:10.1042/0264-6021:3630195. PMC 1222467. PMID 11903063.
- Gigoux V, L'Hoste S, Raynaud F, Camonis J, Garbay C (2002). "Identification of Aurora kinases as RasGAP Src homology 3 domain-binding proteins". J. Biol. Chem. 277 (26): 23742–6. doi:10.1074/jbc.C200121200. PMID 11976319.
- Kufer TA, Silljé HH, Körner R, Gruss OJ, Meraldi P, Nigg EA (2002). "Human TPX2 is required for targeting Aurora-A kinase to the spindle". J. Cell Biol. 158 (4): 617–23. doi:10.1083/jcb.200204155. PMC 2174010. PMID 12177045.
- Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS (2002). "Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function". EMBO J. 21 (17): 4491–9. doi:10.1093/emboj/cdf409. PMC 126178. PMID 12198151.
External links
- Human AURKA genome location and AURKA gene details page in the UCSC Genome Browser.
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Aurora kinase A
- PDBe-KB provides an overview of all the structure information available in the PDB for Mouse Aurora kinase A