PTPN11
Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.[5][6]
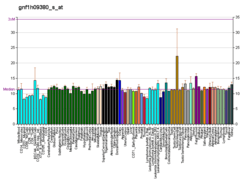
PTPN11 is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. This PTP contains two tandem Src homology-2 domains, which function as phospho-tyrosine binding domains and mediate the interaction of this PTP with its substrates. This PTP is widely expressed in most tissues and plays a regulatory role in various cell signaling events that are important for a diversity of cell functions, such as mitogenic activation, metabolic control, transcription regulation, and cell migration. Mutations in this gene are a cause of Noonan syndrome as well as acute myeloid leukemia.[7]
Structure and function
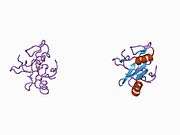
This phosphatase, along with its paralogue, Shp1, possesses a domain structure that consists of two tandem SH2 domains in its N-terminus followed by a protein tyrosine phosphatase (PTP) domain. In the inactive state, the N-terminal SH2 domain binds the PTP domain and blocks access of potential substrates to the active site. Thus, Shp2 is auto-inhibited.
Upon binding to target phospho-tyrosyl residues, the N-terminal SH2 domain is released from the PTP domain, catalytically activating the enzyme by relieving this auto-inhibition.
Genetic diseases associated with PTPN11
Missense mutations in the PTPN11 locus are associated with both Noonan syndrome and Leopard syndrome.
It has also been associated with Metachondromatosis.[8]
Noonan syndrome
In the case of Noonan syndrome, mutations are broadly distributed throughout the coding region of the gene but all appear to result in hyper-activated, or unregulated mutant forms of the protein. Most of these mutations disrupt the binding interface between the N-SH2 domain and catalytic core necessary for the enzyme to maintain its auto-inhibited conformation.[9]
Leopard syndrome
The mutations that cause Leopard syndrome are restricted regions affecting the catalytic core of the enzyme producing catalytically impaired Shp2 variants.[10] It is currently unclear how mutations that give rise to mutant variants of Shp2 with biochemically opposite characteristics result in similar human genetic syndromes.
Cancer associated with PTPN11
Patients with a subset of Noonan syndrome PTPN11 mutations also have a higher prevalence of juvenile myelomonocytic leukemias (JMML). Activating Shp2 mutations have also been detected in neuroblastoma, melanoma, acute myeloid leukemia, breast cancer, lung cancer, colorectal cancer.[11] Recently, a relatively high prevalence of PTPN11 mutations (24%) were detected by next-generation sequencing in a cohort of NPM1-mutated acute myeloid leukemia patients,[12] although the prognostic significance of such associations has not been clarified. These data suggests that Shp2 may be a proto-oncogene. However, it has been reported that PTPN11/Shp2 can act as either tumor promoter or suppressor.[13] In aged mouse model, hepatocyte-specific deletion of PTPN11/Shp2 promotes inflammatory signaling through the STAT3 pathway and hepatic inflammation/necrosis, resulting in regenerative hyperplasia and spontaneous development of tumors. Decreased PTPN11/Shp2 expression was detected in a subfraction of human hepatocellular carcinoma (HCC) specimens.[13] The bacterium Helicobacter pylori has been associated with gastric cancer, and this is thought to be mediated in part by the interaction of its virulence factor CagA with SHP2.[14]
Interactions
PTPN11 has been shown to interact with
- CagA,[14]
- Cbl gene,[15]
- CD117,[16][17]
- CD31,[18][19][20][21]
- CEACAM1,[22]
- Epidermal growth factor receptor,[23][24]
- Erk[25][26]
- FRS2,[27][28][29]
- GAB1,[30][31]
- GAB2,[32][33][34][35]
- GAB3,[36]
- Glycoprotein 130,[37][38][39]
- Grb2,[29][40][41][42][43][44][45][46][47]
- Growth hormone receptor,[48][49]
- HoxA10,[50]
- Insulin receptor,[51][52]
- Insulin-like growth factor 1 receptor,[53][54]
- IRS1,[55][56]
- Janus kinase 1,[37][40]
- Janus kinase 2,[40][57][58]
- LAIR1,[59][60]
- LRP1,[61]
- PDGFRB,[62][63]
- PI3K → Akt[25]
- PLCG2,[32]
- PTK2B,[64]
- Ras[25][26]
- SLAMF1,[65][66]
- SOCS3,[37]
- SOS1,[29][67]
- STAT3,[13]
- STAT5A,[68][69] and
- STAT5B.[68]
H Pylori CagA virulence factor
CagA is a protein and virulence factor inserted by Helicobacter pylori into gastric epithelia. Once activated by SRC phosphorylation, CagA binds to SHP2, allosterically activating it. This leads to morphological changes, abnormal mitogenic signals and sustained activity can result in apoptosis of the host cell. Epidemiological studies have shown roles of cagA- positive H. pylori in the development of atrophic gastritis, peptic ulcer disease and gastric carcinoma.[70]
References
- GRCh38: Ensembl release 89: ENSG00000179295 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000043733 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Jamieson CR, van der Burgt I, Brady AF, van Reen M, Elsawi MM, Hol F, Jeffery S, Patton MA, Mariman E (December 1994). "Mapping a gene for Noonan syndrome to the long arm of chromosome 12". Nat. Genet. 8 (4): 357–60. doi:10.1038/ng1294-357. PMID 7894486.
- Freeman RM, Plutzky J, Neel BG (December 1992). "Identification of a human Src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew". Proc. Natl. Acad. Sci. U.S.A. 89 (23): 11239–43. doi:10.1073/pnas.89.23.11239. PMC 50525. PMID 1280823.
- "Entrez Gene: PTPN11 protein tyrosine phosphatase, non-receptor type 11 (Noonan syndrome 1)".
- Sobreira NL, Cirulli ET, Avramopoulos D, Wohler E, Oswald GL, Stevens EL, Ge D, Shianna KV, Smith JP, Maia JM, Gumbs CE, Pevsner J, Thomas G, Valle D, Hoover-Fong JE, Goldstein DB (June 2010). "Whole-genome sequencing of a single proband together with linkage analysis identifies a Mendelian disease gene". PLoS Genet. 6 (6): e1000991. doi:10.1371/journal.pgen.1000991. PMC 2887469. PMID 20577567.
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Li L, Yassin Y, Tamburino AM, Neel BG, Kucherlapati RS (January 2007). "Germline gain-of-function mutations in SOS1 cause Noonan syndrome". Nat. Genet. 39 (1): 70–4. doi:10.1038/ng1926. PMID 17143285.
- Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG (March 2006). "PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects". J. Biol. Chem. 281 (10): 6785–92. doi:10.1074/jbc.M513068200. PMID 16377799.
- Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ, Richards WG, Du J, Girard L, Minna JD, Loh ML, Fisher DE, Velculescu VE, Vogelstein B, Meyerson M, Sellers WR, Neel BG (December 2004). "Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia". Cancer Res. 64 (24): 8816–20. doi:10.1158/0008-5472.CAN-04-1923. PMID 15604238.
- Patel SS, Kuo FC, Gibson CJ, Steensma DP, Soiffer RJ, Alyea EP, Chen YA, Fathi AT, Graubert TA, Brunner AM, Wadleigh M, Stone RM, DeAngelo DJ, Nardi V, Hasserjian RP, Weinberg OK (May 2018). "High NPM1 mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML". Blood. 131 (25): 2816–2825. doi:10.1182/blood-2018-01-828467. PMC 6265642. PMID 29724895.
- Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V, Varki NM, Wang H, Feng GS (May 2011). "Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis". Cancer Cell. 19 (5): 629–39. doi:10.1016/j.ccr.2011.03.023. PMC 3098128. PMID 21575863.
- Hatakeyama M, Higashi H (2005). "Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis". Cancer Science. 96 (12): 835–843. doi:10.1111/j.1349-7006.2005.00130.x. PMID 16367902.
- Tanaka Y, Tanaka N, Saeki Y, Tanaka K, Murakami M, Hirano T, Ishii N, Sugamura K (August 2008). "c-Cbl-dependent monoubiquitination and lysosomal degradation of gp130". Mol. Cell. Biol. 28 (15): 4805–18. doi:10.1128/MCB.01784-07. PMC 2493370. PMID 18519587.
- Tauchi T, Feng GS, Marshall MS, Shen R, Mantel C, Pawson T, Broxmeyer HE (October 1994). "The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells". J. Biol. Chem. 269 (40): 25206–11. PMID 7523381.
- Kozlowski M, Larose L, Lee F, Le DM, Rottapel R, Siminovitch KA (April 1998). "SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain". Mol. Cell. Biol. 18 (4): 2089–99. doi:10.1128/MCB.18.4.2089. PMC 121439. PMID 9528781.
- Ilan N, Cheung L, Pinter E, Madri JA (July 2000). "Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation". J. Biol. Chem. 275 (28): 21435–43. doi:10.1074/jbc.M001857200. PMID 10801826.
- Pumphrey NJ, Taylor V, Freeman S, Douglas MR, Bradfield PF, Young SP, Lord JM, Wakelam MJ, Bird IN, Salmon M, Buckley CD (April 1999). "Differential association of cytoplasmic signalling molecules SHP-1, SHP-2, SHIP and phospholipase C-gamma1 with PECAM-1/CD31". FEBS Lett. 450 (1–2): 77–83. doi:10.1016/S0014-5793(99)00446-9. PMID 10350061.
- Hua CT, Gamble JR, Vadas MA, Jackson DE (October 1998). "Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates". J. Biol. Chem. 273 (43): 28332–40. doi:10.1074/jbc.273.43.28332. PMID 9774457.
- Jackson DE, Ward CM, Wang R, Newman PJ (March 1997). "The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling". J. Biol. Chem. 272 (11): 6986–93. doi:10.1074/jbc.272.11.6986. PMID 9054388.
- Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N (January 1999). "The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells". J. Biol. Chem. 274 (1): 335–44. doi:10.1074/jbc.274.1.335. PMID 9867848.
- Schulze WX, Deng L, Mann M (2005). "Phosphotyrosine interactome of the ErbB-receptor kinase family". Mol. Syst. Biol. 1 (1): E1–E13. doi:10.1038/msb4100012. PMC 1681463. PMID 16729043.
- Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Böhmer FD (September 1995). "Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C". J. Biol. Chem. 270 (36): 21277–84. doi:10.1074/jbc.270.36.21277. PMID 7673163.
- L.A. Lai; C. Zhao; E.E. Zhang; G.S. Feng (2004). "14 The Shp-2 tyrosine phosphatase". In Joaquín Ariño; Denis Alexander (eds.). Protein phosphatases. Springer. pp. 275–299. ISBN 978-3-540-20560-9.
- Neel BG, Gu H, Pao L (June 2003). "The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling". Trends in Biochemical Sciences. 28 (6): 284–293. doi:10.1016/S0968-0004(03)00091-4. ISSN 0968-0004. PMID 12826400.
- Delahaye L, Rocchi S, Van Obberghen E (February 2000). "Potential involvement of FRS2 in insulin signaling". Endocrinology. 141 (2): 621–8. doi:10.1210/endo.141.2.7298. PMID 10650943.
- Kurokawa K, Iwashita T, Murakami H, Hayashi H, Kawai K, Takahashi M (April 2001). "Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction". Oncogene. 20 (16): 1929–38. doi:10.1038/sj.onc.1204290. PMID 11360177.
- Hadari YR, Kouhara H, Lax I, Schlessinger J (July 1998). "Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation". Mol. Cell. Biol. 18 (7): 3966–73. doi:10.1128/MCB.18.7.3966. PMC 108981. PMID 9632781.
- Saito Y, Hojo Y, Tanimoto T, Abe J, Berk BC (June 2002). "Protein kinase C-alpha and protein kinase C-epsilon are required for Grb2-associated binder-1 tyrosine phosphorylation in response to platelet-derived growth factor". J. Biol. Chem. 277 (26): 23216–22. doi:10.1074/jbc.M200605200. PMID 11940581.
- Rocchi S, Tartare-Deckert S, Murdaca J, Holgado-Madruga M, Wong AJ, Van Obberghen E (July 1998). "Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor-signaling molecules". Mol. Endocrinol. 12 (7): 914–23. doi:10.1210/mend.12.7.0141. PMID 9658397.
- Boudot C, Kadri Z, Petitfrère E, Lambert E, Chrétien S, Mayeux P, Haye B, Billat C (October 2002). "Phosphatidylinositol 3-kinase regulates glycosylphosphatidylinositol hydrolysis through PLC-gamma(2) activation in erythropoietin-stimulated cells". Cell. Signal. 14 (10): 869–78. doi:10.1016/S0898-6568(02)00036-0. PMID 12135708.
- Lynch DK, Daly RJ (January 2002). "PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2". EMBO J. 21 (1–2): 72–82. doi:10.1093/emboj/21.1.72. PMC 125816. PMID 11782427.
- Zhao C, Yu DH, Shen R, Feng GS (July 1999). "Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1". J. Biol. Chem. 274 (28): 19649–54. doi:10.1074/jbc.274.28.19649. PMID 10391903.
- Crouin C, Arnaud M, Gesbert F, Camonis J, Bertoglio J (April 2001). "A yeast two-hybrid study of human p97/Gab2 interactions with its SH2 domain-containing binding partners". FEBS Lett. 495 (3): 148–53. doi:10.1016/S0014-5793(01)02373-0. PMID 11334882.
- Wolf, I.; Jenkins, B. J.; Liu, Y.; Seiffert, M.; Custodio, J. M.; Young, P.; Rohrschneider, L. R. (2002). "Gab3, a New DOS/Gab Family Member, Facilitates Macrophage Differentiation". Molecular and Cellular Biology. 22 (1): 231–244. doi:10.1128/MCB.22.1.231-244.2002. ISSN 0270-7306. PMC 134230. PMID 11739737.
and associates transiently with the SH2 domain-containing proteins p85 and SHP2
- Lehmann U, Schmitz J, Weissenbach M, Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Schneider-Mergener J, Yoshimura A, Neel BG, Heinrich PC, Schaper F (January 2003). "SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130". J. Biol. Chem. 278 (1): 661–71. doi:10.1074/jbc.M210552200. PMID 12403768.
- Anhuf D, Weissenbach M, Schmitz J, Sobota R, Hermanns HM, Radtke S, Linnemann S, Behrmann I, Heinrich PC, Schaper F (September 2000). "Signal transduction of IL-6, leukemia-inhibitory factor, and oncostatin M: structural receptor requirements for signal attenuation". Journal of Immunology. 165 (5): 2535–43. doi:10.4049/jimmunol.165.5.2535. PMID 10946280.
- Kim H, Baumann H (December 1997). "Transmembrane domain of gp130 contributes to intracellular signal transduction in hepatic cells". J. Biol. Chem. 272 (49): 30741–7. doi:10.1074/jbc.272.49.30741. PMID 9388212.
- Yin T, Shen R, Feng GS, Yang YC (January 1997). "Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases". J. Biol. Chem. 272 (2): 1032–7. doi:10.1074/jbc.272.2.1032. PMID 8995399.
- Ganju RK, Brubaker SA, Chernock RD, Avraham S, Groopman JE (June 2000). "Beta-chemokine receptor CCR5 signals through SHP1, SHP2, and Syk". J. Biol. Chem. 275 (23): 17263–8. doi:10.1074/jbc.M000689200. PMID 10747947.
- Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG (July 1994). "Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras". Proc. Natl. Acad. Sci. U.S.A. 91 (15): 7335–9. doi:10.1073/pnas.91.15.7335. PMC 44394. PMID 8041791.
- Ward AC, Monkhouse JL, Hamilton JA, Csar XF (November 1998). "Direct binding of Shc, Grb2, SHP-2 and p40 to the murine granulocyte colony-stimulating factor receptor". Biochim. Biophys. Acta. 1448 (1): 70–6. doi:10.1016/S0167-4889(98)00120-7. PMID 9824671.
- Tang J, Feng GS, Li W (October 1997). "Induced direct binding of the adapter protein Nck to the GTPase-activating protein-associated protein p62 by epidermal growth factor". Oncogene. 15 (15): 1823–32. doi:10.1038/sj.onc.1201351. PMID 9362449.
- Tang H, Zhao ZJ, Huang XY, Landon EJ, Inagami T (April 1999). "Fyn kinase-directed activation of SH2 domain-containing protein-tyrosine phosphatase SHP-2 by Gi protein-coupled receptors in Madin-Darby canine kidney cells". J. Biol. Chem. 274 (18): 12401–7. doi:10.1074/jbc.274.18.12401. PMID 10212213.
- Zhang S, Mantel C, Broxmeyer HE (March 1999). "Flt3 signaling involves tyrosyl-phosphorylation of SHP-2 and SHIP and their association with Grb2 and Shc in Baf3/Flt3 cells". J. Leukoc. Biol. 65 (3): 372–80. doi:10.1002/jlb.65.3.372. PMID 10080542.
- Wong L, Johnson GR (August 1996). "Epidermal growth factor induces coupling of protein-tyrosine phosphatase 1D to GRB2 via the COOH-terminal SH3 domain of GRB2". J. Biol. Chem. 271 (35): 20981–4. doi:10.1074/jbc.271.35.20981. PMID 8702859.
- Stofega MR, Herrington J, Billestrup N, Carter-Su C (September 2000). "Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B". Mol. Endocrinol. 14 (9): 1338–50. doi:10.1210/me.14.9.1338. PMID 10976913.
- Moutoussamy S, Renaudie F, Lago F, Kelly PA, Finidori J (June 1998). "Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins". J. Biol. Chem. 273 (26): 15906–12. doi:10.1074/jbc.273.26.15906. PMID 9632636.
- Wang H, Lindsey S, Konieczna I, Bei L, Horvath E, Huang W, Saberwal G, Eklund EA (January 2009). "Constitutively active SHP2 cooperates with HoxA10 overexpression to induce acute myeloid leukemia". J Biol Chem. 284 (4): 2549–67. doi:10.1074/jbc.M804704200. PMC 2629090. PMID 19022774.
- Maegawa H, Ugi S, Adachi M, Hinoda Y, Kikkawa R, Yachi A, Shigeta Y, Kashiwagi A (March 1994). "Insulin receptor kinase phosphorylates protein tyrosine phosphatase containing Src homology 2 regions and modulates its PTPase activity in vitro". Biochem. Biophys. Res. Commun. 199 (2): 780–5. doi:10.1006/bbrc.1994.1297. PMID 8135823.
- Kharitonenkov A, Schnekenburger J, Chen Z, Knyazev P, Ali S, Zwick E, White M, Ullrich A (December 1995). "Adapter function of protein-tyrosine phosphatase 1D in insulin receptor/insulin receptor substrate-1 interaction". J. Biol. Chem. 270 (49): 29189–93. doi:10.1074/jbc.270.49.29189. PMID 7493946.
- Mañes S, Mira E, Gómez-Mouton C, Zhao ZJ, Lacalle RA, Martínez-A C (April 1999). "Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility". Mol. Cell. Biol. 19 (4): 3125–35. doi:10.1128/mcb.19.4.3125. PMC 84106. PMID 10082579.
- Seely BL, Reichart DR, Staubs PA, Jhun BH, Hsu D, Maegawa H, Milarski KL, Saltiel AR, Olefsky JM (August 1995). "Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp, and GTPase activating protein". J. Biol. Chem. 270 (32): 19151–7. doi:10.1074/jbc.270.32.19151. PMID 7642582.
- Kuhné MR, Pawson T, Lienhard GE, Feng GS (June 1993). "The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp". J. Biol. Chem. 268 (16): 11479–81. PMID 8505282.
- Myers MG, Mendez R, Shi P, Pierce JH, Rhoads R, White MF (October 1998). "The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling". J. Biol. Chem. 273 (41): 26908–14. doi:10.1074/jbc.273.41.26908. PMID 9756938.
- Tauchi T, Damen JE, Toyama K, Feng GS, Broxmeyer HE, Krystal G (June 1996). "Tyrosine 425 within the activated erythropoietin receptor binds Syp, reduces the erythropoietin required for Syp tyrosine phosphorylation, and promotes mitogenesis". Blood. 87 (11): 4495–501. doi:10.1182/blood.V87.11.4495.bloodjournal87114495. PMID 8639815.
- Maegawa H, Kashiwagi A, Fujita T, Ugi S, Hasegawa M, Obata T, Nishio Y, Kojima H, Hidaka H, Kikkawa R (November 1996). "SHPTP2 serves adapter protein linking between Janus kinase 2 and insulin receptor substrates". Biochem. Biophys. Res. Commun. 228 (1): 122–7. doi:10.1006/bbrc.1996.1626. PMID 8912646.
- Fournier N, Chalus L, Durand I, Garcia E, Pin JJ, Churakova T, Patel S, Zlot C, Gorman D, Zurawski S, Abrams J, Bates EE, Garrone P (August 2000). "FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells". Journal of Immunology. 165 (3): 1197–209. doi:10.4049/jimmunol.165.3.1197. PMID 10903717.
- Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH (August 1997). "LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes". Immunity. 7 (2): 283–90. doi:10.1016/S1074-7613(00)80530-0. PMID 9285412.
- Betts GN, van der Geer P, Komives EA (June 2008). "Structural and functional consequences of tyrosine phosphorylation in the LRP1 cytoplasmic domain". J. Biol. Chem. 283 (23): 15656–64. doi:10.1074/jbc.M709514200. PMC 2414285. PMID 18381291.
- Keilhack H, Müller M, Böhmer SA, Frank C, Weidner KM, Birchmeier W, Ligensa T, Berndt A, Kosmehl H, Günther B, Müller T, Birchmeier C, Böhmer FD (January 2001). "Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1". J. Cell Biol. 152 (2): 325–34. doi:10.1083/jcb.152.2.325. PMC 2199605. PMID 11266449.
- Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG (October 1993). "Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor". J. Biol. Chem. 268 (29): 21478–81. PMID 7691811.
- Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, Shima Y, Tai YT, Rosen S, Avraham S, Kharbanda S, Anderson KC (September 2000). "SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells". J. Biol. Chem. 275 (36): 27845–50. doi:10.1074/jbc.M003428200. PMID 10880513.
- Howie D, Simarro M, Sayos J, Guirado M, Sancho J, Terhorst C (February 2000). "Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation". Blood. 99 (3): 957–65. doi:10.1182/blood.V99.3.957. PMID 11806999.
- Morra M, Lu J, Poy F, Martin M, Sayos J, Calpe S, Gullo C, Howie D, Rietdijk S, Thompson A, Coyle AJ, Denny C, Yaffe MB, Engel P, Eck MJ, Terhorst C (November 2001). "Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells". EMBO J. 20 (21): 5840–52. doi:10.1093/emboj/20.21.5840. PMC 125701. PMID 11689425.
- Chin H, Saito T, Arai A, Yamamoto K, Kamiyama R, Miyasaka N, Miura O (October 1997). "Erythropoietin and IL-3 induce tyrosine phosphorylation of CrkL and its association with Shc, SHP-2, and Cbl in hematopoietic cells". Biochem. Biophys. Res. Commun. 239 (2): 412–7. doi:10.1006/bbrc.1997.7480. PMID 9344843.
- Yu CL, Jin YJ, Burakoff SJ (January 2000). "Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation". J. Biol. Chem. 275 (1): 599–604. doi:10.1074/jbc.275.1.599. PMID 10617656.
- Chughtai N, Schimchowitsch S, Lebrun JJ, Ali S (August 2002). "Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells". J. Biol. Chem. 277 (34): 31107–14. doi:10.1074/jbc.M200156200. PMID 12060651.
- Hatakeyama M (September 2004). "Oncogenic mechanisms of the Helicobacter pylori CagA protein". Nature Reviews Cancer. 4 (9): 688–94. doi:10.1038/nrc1433. PMID 15343275.
Further reading
- Marron MB, Hughes DP, McCarthy MJ, Beaumont ER, Brindle NP (2000). Tie-1 receptor tyrosine kinase endodomain interaction with SHP2: potential signalling mechanisms and roles in angiogenesis. Adv. Exp. Med. Biol. Advances in Experimental Medicine and Biology. 476. pp. 35–46. doi:10.1007/978-1-4615-4221-6_3. ISBN 978-1-4613-6895-3. PMID 10949653.
- Carter-Su C, Rui L, Stofega MR (2000). "SH2-B and SIRP: JAK2 binding proteins that modulate the actions of growth hormone". Recent Prog. Horm. Res. 55: 293–311. PMID 11036942.
- Ion A, Tartaglia M, Song X, Kalidas K, van der Burgt I, Shaw AC, Ming JE, Zampino G, Zackai EH, Dean JC, Somer M, Parenti G, Crosby AH, Patton MA, Gelb BD, Jeffery S (2002). "Absence of PTPN11 mutations in 28 cases of cardiofaciocutaneous (CFC) syndrome". Hum. Genet. 111 (4–5): 421–7. doi:10.1007/s00439-002-0803-6. PMID 12384786.
- Hugues L, Cavé H, Philippe N, Pereira S, Fenaux P, Preudhomme C (2006). "Mutations of PTPN11 are rare in adult myeloid malignancies". Haematologica. 90 (6): 853–4. PMID 15951301.
- Tartaglia M, Gelb BD (2005). "Germ-line and somatic PTPN11 mutations in human disease". European Journal of Medical Genetics. 48 (2): 81–96. doi:10.1016/j.ejmg.2005.03.001. PMID 16053901.
- Ogata T, Yoshida R (2006). "PTPN11 mutations and genotype-phenotype correlations in Noonan and LEOPARD syndromes". Pediatric Endocrinology Reviews : PER. 2 (4): 669–74. PMID 16208280.
- Feng GS (2007). "Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation". Cell Res. 17 (1): 37–41. doi:10.1038/sj.cr.7310140. PMID 17211446.
- Edouard T, Montagner A, Dance M, Conte F, Yart A, Parfait B, Tauber M, Salles JP, Raynal P (2007). "How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms?". Cell. Mol. Life Sci. 64 (13): 1585–90. doi:10.1007/s00018-007-6509-0. PMID 17453145.