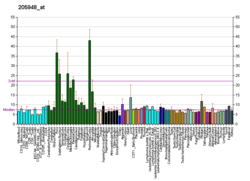
PTPRT
Receptor-type tyrosine-protein phosphatase T is an enzyme that in humans is encoded by the PTPRT gene.[5][6][7]
PTPRT is also known as PTPrho, PTPρ and human accelerated region 9. The human accelerated regions are 49 regions of the human genome that are conserved among vertebrates, but in humans show significant distinction from other vertebrates. This region may, therefore, have played a key role in differentiating humans from apes.[8]
Function
The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. PTPrho has been proposed to function during development of the nervous system and as a tumor suppressor in cancer.
Structure
This PTP possesses an extracellular region, a single transmembrane region, and two tandem intracellular catalytic domains, and thus represents a receptor-type PTP (RPTP). The extracellular region contains a meprin-A5 antigen-PTPmu (MAM) domain, one Ig-like domain and four fibronectin type III-like repeats. PTPrho is a member of the type R2B subfamily of RPTPs, which also includes the RPTPs PTPmu (PTPRM), PTPkappa (PTPRK), and PCP-2 (PTPRU). Comparison of R2B cDNA sequences identified that PTPmu is most closely related to PTPrho.[9] PTPrho is alternatively spliced.[9][10] Alternative splicing of exons 14, 16, and 22a have been described for PTPrho (PTPRT).[10] Two alternatively spliced transcript variants of this gene, which encode distinct proteins, have been reported.[7] The first isoform encodes the larger version of the protein. The second variant lacks a region of the extracellular domain between the fourth FNIII domain and the transmembrane domain and in the juxtamembrane domain.[7]
Homophilic binding
PTPrho protein mediates homophilic cell-cell adhesion, meaning that when it interacts with a like molecule on an adjacent cell it induces the cells to bind or adhere to one another.[11] PTPrho does not bind to other subfamily members to mediate cell-cell aggregation, similar to other type R2B subfamily members.[11][12]
The MAM domain, Ig domain and all four fibronectin III domain of PTPrho are necessary for cell-cell aggregation.[11][12] PTPrho is the most frequently mutated RPTP in colon, lung, skin and stomach cancers.[13] Many of the mutations observed in cancer occur in the extracellular domain of PTPrho, suggesting that defective cell-cell aggregation may contribute to the tumorigenicity of these mutations.[13] When PTPrho proteins are engineered with the different point mutations observed in cancer and then are expressed in non-adherent Sf9 cells, these cells do not mediate comparable levels of cell-cell aggregation to wild-type PTPrho, demonstrating that the mutations observed in cancer are loss of function mutations.[11][12]
Tyrosine phosphatase activity
The first catalytic domain of Type R2B RPTPs is considered the active phosphatase domain, whereas the second phosphatase domain is thought to be inactive.[14] Mutations in the second phosphatase domain of PTPrho, however, result in a reduction of phosphatase activity of PTPrho.[13] Deletion of the second tyrosine phosphatase domain in colorectal cancer cells also reduces PTPrho catalytic activity, again demonstrating that the second phosphatase domain of PTPrho does regulate catalytic activity, either directly or indirectly.[15]
Catalytic activity of PTPrho may also be regulated by tyrosine phosphorylation of the wedge domain of the first tyrosine phosphatase domain on tyrosine 912 by Fyn tyrosine kinase.[16] Tyrosine phosphorylation of Y912 results in increased multimerization of PTPrho, likely in cis, with other PTPrho molecules. Based on crystal structure analysis and modeling, the phosphorylated wedge domain is hypothesized to insert into the catalytic domain of a neighboring PTPrho molecule, thus inactivating it.[16] This mechanism has also been proposed to regulate the catalytic activity of RPTPalpha.[17] The crystal structures of PTPmu and LAR suggest a different mechanism for the regulation of their catalytic activity, as these RPTPs are in an open and active conformation when dimerized.[18]
Regulation of gene expression
Evaluation of the 5’untranslated regions of PTPrho (PTPRT) cDNA indicate a number of transcription factor binding site consensus sequences, including those for AP-2, c-Myb, NF-1, sox-5, and Sp-1, Oct-1, CdxA, C/EBP, En-1, GATA-1, GATA-2, GKLF, HoxA3, Ik-2, Msx-1, Pax-4 and SRY.[9]
(RE1-silencing transcription factor) (REST) is a transcription repressor that binds to REST DNA recognition element (RE-1) in 5’UTRs. A screen of single nucleotide polymorphic genetic changes within the REST binding regions of DNA sequences revealed a polymorphism in the RE-1 of PTPrho (PTPRT). This SNP would result in less REST repressor activity, which could lead to increased expression of PTPrho (PTPRT) in cells that harbored this SNP.[19]
Expression and function in cancer
PTPrho is the most frequently mutated RPTP in colon, lung, skin and stomach cancers.[13] Evaluation of the cytoplasmic mutations observed in PTPrho in cancer demonstrate that they all reduce catalytic activity, even the mutations located in the second catalytic domain.[13] The frequency of mutations in the cytoplasmic tyrosine phosphatase domain of PTPrho in cancer has been disputed.[20] The PTPrho (PTPRT) promoter was observed to be hypermethylated in colorectal cancer compared to controls, suggesting another mechanism whereby PTPrho function may be reduced in cancer, in this instance by epigenetic silencing.[21]
PTPrho is also upregulated in estrogen receptor alpha positive breast tumor samples versus estrogen receptor alpha negative tumor samples.[22] The authors evaluated 560 selected genes by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) in estrogen receptor alpha positive tissue and compared it to estrogen receptor alpha negative tissue, and found that PTPrho(PTPRT) was upregulated in the estrogen receptor alpha tissue, suggesting a non-tumor suppressor role for PTPrho.[22]
Expression and function in the developing nervous system
PTPrho (PTPRT) mRNA is expressed in the developing nervous system.[5][6][23] Its expression is first observed in stage 25 in Xenopus embryos in the developing optic vesicles and in nascent motor and interneurons of the spinal cord.[23] At stage 35/36, PTPrho (PTPRT) expression is found in the outer nuclear, or photoreceptor, layer, and in the inner nuclear layer (INL) of the neural retina. The level of PTPrho (PTPRT) transcript decreases in the photoreceptors and increases in the INL, and by stage 41, is restricted to the INL only.[23] PTPrho (PTPRT) transcripts have also been observed in the developing cortex and olfactory bulbs.[6]
PTPrho (PTPRT) is expressed in a very specific subset of neurons in the postnatal cerebellar cortex, the granule cell layer. Specifically, PTPrho (PTPRT) was expressed in postmigratory granule cells of lobules 1 to 6 of the cerebellum.[5]
In adults, PTPrho protein is exclusively expressed in the central nervous system and localizes to synapses between neurons.[16] Over-expression of wild-type and catalytically inactive mutant forms of PTPrho result in an increase in the number of excitatory and inhibitory synapses in cultured neurons in vitro. Knock-down of PTPrho expression decreases the number of synapses in cultured neurons. PTPrho interacts in cis with the extracellular domains of neuroligins and neurexins at synapses.[16] PTPrho is phosphorylated on tyrosine 912 in the wedge region of its first catalytic domain by Fyn tyrosine kinase. Phosphorylation at this site attenuates synapse formation in cultured neurons. When PTPrho is phosphorylated by Fyn, PTPrho appears to form homophilic multimerizations, likely in cis, which appear to decrease PTPrho association with neuroligins and neurexins. The reduction of cis interactions with neuroligins and neurexons is hypothesized to ultimately lead to the reduction in synapse formation.[16]
PTPrho activity has also been demonstrated to be required for the development of neuronal dendrites. It was found to regulate dendritic arborization by dephosphorylating tyrosine 177 of Breakpoint cluster region protein (BCR).[24]
Substrates
PTPrho associates with members of the cadherin and catenin family of cell adhesion molecules as demonstrated by GST-fusion protein pull-down assays using brain homogenate. Using this technique, the authors identified that PTPrho interacts with alpha-actinin, alpha-catenin, beta-catenin, gamma-catenin/plakoglobin, p120 catenin, desmoglein, E-cadherin, N-cadherin, and VE-cadherin.[25] Purified wild-type PTPrho GST fusion protein was able to dephosphorylate E-cadherin and p120catenin co-immunoprecipitated from a pancreatic beta cell line, MIN6-m9. This suggests that these proteins are PTPrho substrates.[25]
PTPrho also dephosphorylates BCR protein.[24] The ability of PTPrho to dephosphorylate BCR was shown to have functional consequences for the normal development of neuronal dendritic arborization.
PTPrho dephosphorylates STAT3, signal transducer and activator of transcription 3, on tyrosine 705, a residue that is critical for the activation of STAT3.[15] Dephosphorylation by PTPrho in colorectal cancer cells results in a reduction in the total level of transcription of the STAT3 target genes, Bcl-XL and SOCS3. Likewise, expression of wild-type PTPrho decreases the ability of STAT3 to translocate to the nucleus, where it needs to localize to function as a transcription factor.[15]
PTPrho also dephosphorylates paxillin on tyrosine 88.[26] Higher levels of tyrosine 88 phosphorylation of paxillin are observed in colon cancers. When colon cancer cells are engineered to express a mutant form of paxillin that is incapable of being tyrosine phosphorylated, the paxillin Y88F mutant, these cells exhibit reduced tumorigenicity. This suggests that PTPrho may function as a tumor suppressor protein by regulating paxillin phosphorylation.[26]
Interacting proteins
PTPrho has been shown to interact with:
- alpha-actinin[25]
- Alpha catenin[25]
- Beta-catenin[25]
- Breakpoint cluster region protein (BCR)[24]
- Desmoglein[25]
- E-cadherin[25]
- Fyn[16]
- N-cadherin[25]
- gamma-catenin[25]
- p120-catenin[25]
- Paxillin[26]
- Neuroligin[16]
- Neurexin[16]
- STAT3[15]
- VE-cadherin/Cadherin-5 [25]
References
- GRCh38: Ensembl release 89: ENSG00000196090 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000053141 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- McAndrew PE, Frostholm A, Evans JE, Zdilar D, Goldowitz D, Chiu IM, Burghes AH, Rotter A (Mar 1998). "Novel receptor protein tyrosine phosphatase (RPTPrho) and acidic fibroblast growth factor (FGF-1) transcripts delineate a rostrocaudal boundary in the granule cell layer of the murine cerebellar cortex". J Comp Neurol. 391 (4): 444–55. doi:10.1002/(SICI)1096-9861(19980222)391:4<444::AID-CNE3>3.0.CO;2-0. PMID 9486824.
- McAndrew PE, Frostholm A, White RA, Rotter A, Burghes AH (Jan 1999). "Identification and characterization of RPTP rho, a novel RPTP mu/kappa-like receptor protein tyrosine phosphatase whose expression is restricted to the central nervous system". Brain Res Mol Brain Res. 56 (1–2): 9–21. doi:10.1016/S0169-328X(98)00014-X. PMID 9602027.
- "Entrez Gene: PTPRT protein tyrosine phosphatase, receptor type, T".
- Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A, Kern AD, Dehay C, Igel H, Ares M, Vanderhaeghen P, Haussler D (September 2006). "An RNA gene expressed during cortical development evolved rapidly in humans". Nature. 443 (7108): 167–72. doi:10.1038/nature05113. PMID 16915236. supplement
- Besco J, Popesco MC, Davuluri RV, Frostholm A, Rotter A (2004). "Genomic structure and alternative splicing of murine R2B receptor protein tyrosine phosphatases (PTPkappa, mu, rho and PCP-2)". BMC Genomics. 5 (1): 14. doi:10.1186/1471-2164-5-14. PMC 373446. PMID 15040814.
- Besco JA, Frostholm A, Popesco MC, Burghes AH, Rotter A (2001). "Genomic organization and alternative splicing of the human and mouse RPTPrho genes". BMC Genomics. 2: 1. doi:10.1186/1471-2164-2-1. PMC 33392. PMID 11423001.
- Yu J, Becka S, Zhang P, Zhang X, Brady-Kalnay SM, Wang Z (2008). "Tumor-derived extracellular mutations of PTPRT /PTPrho are defective in cell adhesion". Mol Cancer Res. 6 (7): 1106–13. doi:10.1158/1541-7786.MCR-07-2123. PMC 2614372. PMID 18644975.
- Zhang P, Becka S, Craig SE, Lodowski DT, Brady-Kalnay SM, Wang Z (2009). "Cancer-derived mutations in the fibronectin III repeats of PTPRT/PTPrho inhibit cell-cell aggregation". Cell Commun Adhes. 16 (5–6): 146–53. doi:10.3109/15419061003653771. PMC 2921943. PMID 20230342.
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, et al. (2004). "Mutational analysis of the tyrosine phosphatome in colorectal cancers". Science. 304 (5674): 1164–6. doi:10.1126/science.1096096. PMID 15155950.
- Neel BG, Tonks NK (1997). "Protein tyrosine phosphatases in signal transduction". Curr Opin Cell Biol. 9 (2): 193–204. doi:10.1016/S0955-0674(97)80063-4. PMID 9069265.
- Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W, et al. (2007). "Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T". Proc Natl Acad Sci U S A. 104 (10): 4060–4. doi:10.1073/pnas.0611665104. PMC 1802729. PMID 17360477.
- Lim SH, Kwon SK, Lee MK, Moon J, Jeong DG, Park E, et al. (2009). "Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn". EMBO J. 28 (22): 3564–78. doi:10.1038/emboj.2009.289. PMC 2782100. PMID 19816407.
- Bilwes AM, den Hertog J, Hunter T, Noel JP (1996). "Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization". Nature. 382 (6591): 555–9. doi:10.1038/382555a0. PMID 8700232.
- Ensslen-Craig SE, Brady-Kalnay SM (2004). "Receptor protein tyrosine phosphatases regulate neural development and axon guidance". Dev Biol. 275 (1): 12–22. doi:10.1016/j.ydbio.2004.08.009. PMID 15464569.
- Johnson R, Richter N, Bogu GK, Bhinge A, Teng SW, Choo SH, et al. (2012). "A genome-wide screen for genetic variants that modify the recruitment of REST to its target genes". PLOS Genet. 8 (4): e1002624. doi:10.1371/journal.pgen.1002624. PMC 3320604. PMID 22496669.
- Lee JW, Jeong EG, Lee SH, Nam SW, Kim SH, Lee JY, et al. (2007). "Mutational analysis of PTPRT phosphatase domains in common human cancers". APMIS. 115 (1): 47–51. doi:10.1111/j.1600-0463.2007.apm_554.x. PMID 17223850.
- Laczmanska I, Karpinski P, Bebenek M, Sedziak T, Ramsey D, Szmida E, et al. (2013). "Protein tyrosine phosphatase receptor-like genes are frequently hypermethylated in sporadic colorectal cancer" (PDF). J Hum Genet. 58 (1): 11–5. doi:10.1038/jhg.2012.119. PMID 23096495.
- Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, et al. (2006). "Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach". Endocr Relat Cancer. 13 (4): 1109–20. doi:10.1677/erc.1.01120. PMID 17158757.
- Johnson KG, Holt CE (2000). "Expression of CRYP-alpha, LAR, PTP-delta, and PTP-rho in the developing Xenopus visual system". Mech Dev. 92 (2): 291–4. doi:10.1016/S0925-4773(99)00345-7. PMID 10727868.
- Park AR, Oh D, Lim SH, Choi J, Moon J, Yu DY, et al. (2012). "Regulation of dendritic arborization by BCR Rac1 GTPase-activating protein, a substrate of PTPRT". J Cell Sci. 125 (Pt 19): 4518–31. doi:10.1242/jcs.105502. PMID 22767509.
- Besco JA, Hooft van Huijsduijnen R, Frostholm A, Rotter A (2006). "Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT)". Brain Res. 1116 (1): 50–7. doi:10.1016/j.brainres.2006.07.122. PMID 16973135.
- Zhao Y, Zhang X, Guda K, Lawrence E, Sun Q, Watanabe T, et al. (2010). "Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T". Proc Natl Acad Sci U S A. 107 (6): 2592–7. doi:10.1073/pnas.0914884107. PMC 2823898. PMID 20133777.
Further reading
- Laczmanska I, Sasiadek MM (2011). "Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer". Acta Biochim Pol. 58 (4): 467–70. doi:10.18388/abp.2011_2212. PMID 22146137.
- Scott A, Wang Z (2011). "Tumour suppressor function of protein tyrosine phosphatase receptor-T". Biosci Rep. 31 (5): 303–7. doi:10.1042/BSR20100134. PMC 3116232. PMID 21517784.