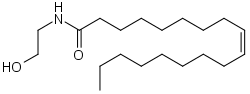
Oleoylethanolamide
Oleoylethanolamine (OEA) is an endogenous peroxisome proliferator-activated receptor alpha (PPAR-α) agonist. It is a naturally occurring ethanolamide lipid that regulates feeding and body weight in vertebrates ranging from mice to pythons.[1][2][3]
 | |
| Names | |
|---|---|
| IUPAC name
(Z)-N-(2-Hydroxyethyl)octadec-9-enamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.532 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H39NO2 | |
| Molar mass | 325.537 g·mol−1 |
| Appearance | White solid |
| Melting point | 59–60 °C (138–140 °F; 332–333 K) |
| Solubility in ethanol and DMSO | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
OEA is a shorter, monounsaturated analogue of the endocannabinoid anandamide, but unlike anandamide it acts independently of the cannabinoid pathway, regulating PPAR-α activity to stimulate lipolysis.[4]
OEA is produced by the small intestine following feeding in two steps. First an N-acyl transferase (NAT) activity joins the free amino terminus of phosphatidylethanolamine (PE) to the oleoyl group (one variety of acyl group) derived from sn-1-oleoyl-phosphatidylcholine, which contains the fatty acid oleic acid at the sn-1 position.[5] This produces an N-acylphosphatidylethanolamine, which is then split (hydrolyzed) by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) into phosphatidic acid and OEA. The biosynthesis of OEA and other bioactive lipid amides is modulated by bile acids.[6]
OEA has been demonstrated to bind to the novel cannabinoid receptor GPR119.[7] OEA has been suggested to be the receptor's endogenous ligand.[8]
OEA has been reported to lengthen the life span of the roundworm Caenorhabditis elegans through interactions with lysomal molecules.[9]
References
- Gaetani S, Oveisi F, Piomelli D (2003). "Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamine". Neuropsychopharmacology. 28 (7): 1311–6. doi:10.1038/sj.npp.1300166. PMID 12700681.
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D (2005). "Regulation of food intake by oleoylethanolamine". Cell. Mol. Life Sci. 62 (6): 708–16. doi:10.1007/s00018-004-4494-0. PMID 15770421.
- Giuseppe Astarita; Bryan C. Rourke; Johnnie B. Andersen; Jin Fu; Janet H. Kim; Albert F. Bennett; James W. Hicks & Daniele Piomelli (2005-12-22). "Postprandial increase of oleoylethanolamine mobilization in small intestine of the Burmese python (Python molurus)". Am J Physiol Regul Integr Comp Physiol. 290 (5): R1407–R1412. doi:10.1152/ajpregu.00664.2005. PMID 16373434.
- Gaetani S, Kaye WH, Cuomo V, Piomelli D (September 2008). "Role of endocannabinoids and their analogues in obesity and eating disorders". Eat Weight Disord. 13 (3): e42–8. PMID 19011363.
- illustration
- Magotti P, Bauer I, Igarashi M, Babagoli M, Marotta R, Piomelli D, Garau G (Dec 2014). "Structure of Human N-Acylphosphatidylethanolamine-Hydrolyzing Phospholipase D: Regulation of Fatty Acid Ethanolamide Biosynthesis by Bile Acids". Structure. 23 (3): 598–604. doi:10.1016/j.str.2014.12.018. PMC 4351732. PMID 25684574.
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C (2006). "Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents". Cell Metab. 3 (3): 167–175. doi:10.1016/j.cmet.2006.02.004. PMID 16517404.
- Brown AJ. (2007). "Novel cannabinoid receptors". Br J Pharmacol. 152 (5): 567–575. doi:10.1038/sj.bjp.0707481. PMC 2190013. PMID 17906678.
- Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, Wang MC (2015). "Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans". Science. 347 (6217): 83–6. doi:10.1126/science.1258857. PMC 4425353. PMID 25554789.