Resiniferatoxin
Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge (Euphorbia resinifera), a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria.[1] It is a potent functional analog of capsaicin, the active ingredient in chili peppers.[2]
 | |
| Names | |
|---|---|
| IUPAC name
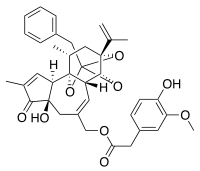
[(1R,6R,13R,15R,17R)-13-Benzyl-6-hydroxy-4,17-dimethyl-5-oxo-15-(prop-1-en-2-yl)-12,14,18-trioxapentacyclo[11.4.1.01,10.02,6.011,15]octadeca-3,8-dien-8-yl]methyl 2-(4-hydroxy-3-methoxyphenyl)acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| MeSH | resiniferatoxin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C37H40O9 | |
| Molar mass | 628.718 g·mol−1 |
| Density | 1.35 ± 0.1 g/cm³ |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Resiniferatoxin | |
|---|---|
| Heat | Above peak (compound is highly toxic however) |
| Scoville scale | 16,000,000,000 SHU |
Biological activity
Resiniferatoxin has a score of 16 billion Scoville heat units, making pure resiniferatoxin about 500 to 1000 times hotter than pure capsaicin.[3][4] Resiniferatoxin activates transient vanilloid receptor 1 (TRPV1) in a subpopulation of primary afferent sensory neurons involved in nociception, the transmission of physiological pain.[5][6] TRPV1 is an ion channel in the plasma membrane of sensory neurons and stimulation by resiniferatoxin causes this ion channel to become permeable to cations, especially calcium. The influx of cations causes the neuron to depolarize, transmitting signals similar to those that would be transmitted if the innervated tissue were being burned or damaged. This stimulation is followed by desensitization and analgesia, in part because the nerve endings die from calcium overload.[7][8]
Total synthesis
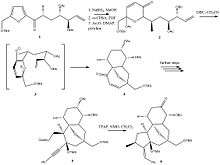
A total synthesis of (+)-resiniferatoxin was completed by the Wender group at Stanford University in 1997.[9] The process begins with a starting material of 1,4-pentadien-3-ol and consists of more than 25 significant steps. As of 2007, this represented the only complete total synthesis of any member of the daphnane family of molecules.[10]
One of the main challenges in synthesizing a molecule such as resiniferatoxin is forming the three-ring backbone of the structure. The Wender group was able to form the first ring of the structure by first synthesizing Structure 1 in Figure 1. By reducing the ketone of Structure 1 followed by oxidizing the furan nucleus with m-CPBA and converting the resulting hydroxy group to an oxyacetate, Structure 2 can be obtained. Structure 2 contains the first ring of the three-ring structure of RTX. It reacts through an oxidopyrylium cycloaddition when heated with DBU in acetonitrile to form Structure 4 by way of Intermediate 3. Several steps of synthesis are required to form Structure 5 from Structure 4, with the main goal of positioning the allylic branch of the seven-membered ring in a trans conformation. Once this conformation is achieved, zirconocene-mediated cyclization of Structure 5 can occur, and oxidizing the resulting hydroxy group with TPAP will yield Structure 6. Structure 6 contains all three rings of the RTX backbone and can then be converted to resiniferatoxin through additional synthesis steps attaching the required functional groups.[9]
An alternative approach to synthesizing the three-ring backbone makes use of radical reactions to create the first and third rings in a single step, followed by the creation of the remaining ring. It has been proposed by the Inoue group of the University of Tokyo.[11]
Toxicity
Resiniferatoxin is rather toxic and can inflict chemical burns in tiny quantities. The primary action of resiniferatoxin is to activate sensory neurons responsible for the perception of pain. It is currently the most potent TRPV1 agonist known, with ~500x higher binding affinity for TRPV1 than capsaicin, the active ingredient in hot chili peppers such as those produced by Capsicum annuum. Animal experiments on the rat suggest that, in humans, ingestion of 1.672 g may be fatal or cause serious damage to health.[12][13] It causes severe burning pain in sub-microgram (less than 1/1,000,000th of a gram) quantities when ingested orally.
Research
Sorrento Therapeutics has been developing RTX as a means to provide pain relief for forms of advanced cancer.[14][15]
The nerve desensitizing properties of RTX were once thought to be useful to treat overactive bladder (OAB) by preventing the bladder from transmitting “sensations of urgency” to the brain, similar to how they can prevent nerves from transmitting signals of pain; RTX has never received FDA approval for this use.[4] RTX has also previously been investigated as a treatment for interstitial cystitis, rhinitis, and lifelong premature ejaculation (PE).[15]
See also
- List of investigational analgesics
- Discovery and development of TRPV1 antagonists
- Iodoresiniferatoxin
- Transient receptor potential
- Tinyatoxin
References
- Euphorbia poissonii in BoDD – Botanical Dermatology Database
- Christopher S. J. Walpole; et al. (1996). "Similarities and Differences in the Structure-Activity Relationships of Capsaicin and Resiniferatoxin Analogues". J. Med. Chem. 39 (15): 2939–2952. doi:10.1021/jm960139d. PMID 8709128.
- National Institutes of Health, Clinical Center Department of Perioperative Medicine Chemical from cactus-like plant shows promise in controlling surgical pain, while leaving touch and coordination intact, rat study shows News release December 21, 2017, retrieved 28 February 2018.
- Ellsworth, Pamela; Wein, Alan J. (2009). Questions and Answers about Overactive Bladder. Jones & Bartlett Learning. pp. 97–100. ISBN 978-1449631130.
- Szallasi A, Blumberg PM (1989). "Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analogue of capsaicin, the irritant constituent in red pepper". Neuroscience. 30 (2): 515–520. doi:10.1016/0306-4522(89)90269-8. PMID 2747924.
- Szallasi A, Blumberg PM (1990). "Resiniferatoxin and its analogues provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor". Life Sci. 47 (16): 1399–1408. doi:10.1016/0024-3205(90)90518-V. PMID 2174484.
- Szallasi A, Blumberg PM (1992). "Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin". Neurosci. Lett. 140 (1): 51–54. doi:10.1016/0304-3940(92)90679-2. PMID 1407700.
- Olah Z, et al. (2001). "Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1". J. Biol. Chem. 276 (14): 11021–11030. doi:10.1074/jbc.M008392200. hdl:2437/104771. PMID 11124944.
- Wender, P.A.; Jesudason, Cynthia D.; Nakahira, Hiroyuki; Tamura, Norikazu; Tebbe, Anne Louise; Ueno, Yoshihide (1997). "The First Synthesis of a Daphnane Diterpene: The Enantiocontrolled Total Synthesis of (+)-Resiniferatoxin". J. Am. Chem. Soc. 119 (52): 12976–12977. doi:10.1021/ja972279y.
- Seiple, I.B. (March 17, 2007). "Daphnane, Tigliane, Ingenane and Lathyrane Diterpenes" (PDF). scripps.edu.
- "Resiniferatoxin– A Radical Approach – Chemical Science Blog". blogs.rsc.org.
- "Material Safety Data Sheet for resiniferatoxin, 2009" (PDF).
- Nair AB, Jacob S (2016). "A simple practice guide for dose conversion between animals and human". J Basic Clin Pharm. 7 (2): 27–31. doi:10.4103/0976-0105.177703. PMC 4804402. PMID 27057123.
- Brown, D.C. (2016). "Resiniferatoxin: The Evolution of the 'Molecular Scalpel' for Chronic Pain Relief". Pharmaceuticals. 9 (3): 47. doi:10.3390/ph9030047. PMC 5039500. PMID 27529257.
- "Resiniferatoxin - Sorrento Therapeutics - AdisInsight". adisinsight.springer.com. 2019-01-24.
External links
- Fiery pepper may hold key to easing pain Lauran Neergaard, The Associated Press Published 10:00 pm PST, Monday, January 16, 2006
- CID Resiniferatoxin from PubChem