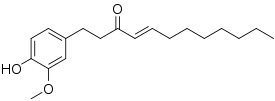
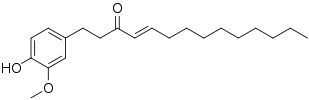
Shogaol
Shogaols are pungent constituents of ginger similar in chemical structure to gingerol. The most common of the group is [6]-shogaol. Like zingerone, it is produced when ginger is dried or cooked.[1] Moreover, shogaol (and gingerol) are converted to other constituents when heat is applied over time, which is why ginger loses its spiciness as it is cooked.
 | |
| Names | |
|---|---|
| IUPAC name
(E)-1-(4-Hydroxy-3- methoxyphenyl)dec-4-en-3-one | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.190.262 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H24O3 | |
| Molar mass | 276.376 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Shogaol | |
|---|---|
| Heat | |
| Scoville scale | 160,000 SHU |
The name shogaol is derived from the Japanese name for ginger (生姜、shōga).
Shogaol is rated 160,000 SHU on the Scoville scale. When compared to other pungent compounds, shogaol is moderately more pungent than piperine, but less than capsaicin.
| Compound | Scoville Heat Units (SHU) |
|---|---|
| Capsaicin | 16,000,000[2] |
| [6]-Shogaol | 160,000 |
| Piperine | 100,000 |
| [6]-Gingerol | 60,000 |
Shogaols group
[4]-Shogaol, [8]-shogaol, [10]-shogaol, and [12]-shogaol (all found in ginger) together constitute the group shogaols. There also exist in ginger cultivars methylated shogaols: methyl [6]-shogaol and methyl [8]-shogaol, respectively.[3]
Shogaols are artifacts formed during storage or through excess heat, probably created by a dehydration reaction of the gingerols. The ratio of shogaols to gingerols sometimes is taken as an indication of product quality.[4]
Synthesis
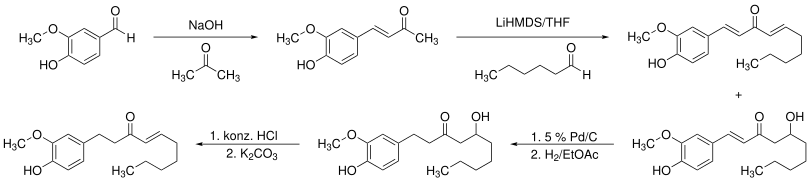
A possible synthesis starts with a claisen condensation of vanillin and acetone, producing dehydrozingerone. Afterwards the product reacts in an aldol condensation with hexanal in tetrahydrofurane to 6-dehydroshogaol and 6-dehydrogingerol. Latter can be hydrogenated to [6]-gingerol by a catalyst. In the last step hydrochloric acid is added to get the desired [6]-shogaol.[5]
References
- Harold McGee (2004). On Food and Cooking: The Science and Lore of the Kitchen (2nd ed.). New York: Scribner. pp. 425–426.
- Govindarajan, Sathyanarayana (1991). "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- "Analysis of Chemical Properties of Edible and Medicinal Ginger by Metabolomics Approach : Table 1". Retrieved 3 December 2016.
- NSF International Determination of Gingerols and Shogaols in Zingiber officinale rhizome and powdered extract by High-Performance Liquid Chromatography
- Hung-Cheng Shih; et al. (March 2014). "Synthesis of Analogues of Gingerol and Shogaol, the Active Pungent Principles from the Rhizomes of Zingiber officinale and Evaluation of Their Anti-Platelet Aggregation Effects". International Journal of Molecular Sciences. 15 (3): 3926–3951. doi:10.3390/ijms15033926. PMC 3975376. PMID 24599082.