Kozak consensus sequence
The Kozak consensus sequence (Kozak consensus or Kozak sequence) is a nucleic acid motif that functions as the protein translation initiation site in most eukaryotic mRNA transcripts.[1] Regarded as the optimum sequence for initiating translation in eukaryotes, the sequence is an integral aspect of protein regulation and overall cellular health as well as having implications in human disease.[1][2] It ensures that a protein is correctly translated from the genetic message, mediating ribosome assembly and translation initiation. A wrong start site can result in non-functional proteins.[3] As it has become more studied, expansions of the nucleotide sequence, bases of importance, and notable exceptions have arisen.[1][4][5] The sequence was named after the scientist who discovered it, Marilyn Kozak. Kozak discovered the sequence through a detailed analysis of DNA genomic sequences.[6]
The Kozak sequence is not to be confused with the ribosomal binding site (RBS), that being either the 5′ cap of a messenger RNA or an internal ribosome entry site (IRES).
Sequence
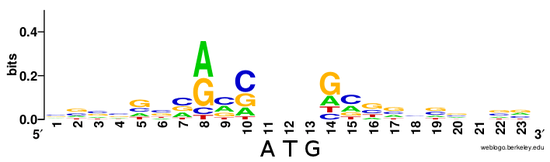
The Kozak Sequence was determined by sequencing of 699 vertebrate mRNAs and verified by site-directed mutagenesis.[7] While initially limited to a subset of vertebrates (i.e. human, cow, cat, dog, chicken, guinea pig, hamster, mouse, pig, rabbit, sheep, and Xenopus), subsequent studies confirmed its conservation in higher eukaryotes generally.[1] The sequence was defined as 5'-(gcc)gccRccAUGG-3(IUPAC nucleobase notation summarized here) where:[7]
- The underlined nucleotides indicate the translation start codon, coding for Methionine.
- upper-case letters indicate highly conserved bases, i.e. the 'AUGG' sequence is constant or rarely, if ever, changes.[8]
- 'R' indicates that a purine (adenine or guanine) is always observed at this position (with adenine being more frequent according to Kozak)
- a lower-case letter denotes the most common base at a position where the base can nevertheless vary
- the sequence in parentheses (gcc) is of uncertain significance.
The AUG is the initiation codon encoding a methionine amino acid at the N-terminus of the protein. (Rarely, GUG is used as an initiation codon, but methionine is still the first amino acid as it is the met-tRNA in the initiation complex that binds to the mRNA). Variation within the Kozak sequence alters the "strength" thereof. Kozak sequence strength refers to the favorability of initiation, affecting how much protein is synthesized from a given mRNA.[4][9] The A nucleotide of the "AUG" is delineated as +1 in mRNA sequences with the preceding base being labeled as −1. For a 'strong' consensus, the nucleotides at positions +4 (i.e. G in the consensus) and −3 (i.e. either A or G in the consensus) relative to the +1 nucleotide must both match the consensus (there is no 0 position). An 'adequate' consensus has only 1 of these sites, while a 'weak' consensus has neither. The cc at −1 and −2 are not as conserved, but contribute to the overall strength.[10] There is also evidence that a G in the -6 position is important in the initiation of translation.[4] While the +4 and the −3 positions in the Kozak sequence have the greatest relative importance in the establishing a favorable initiation context a CC or AA motif at −2 and −1 were found to be important in the initiation of translation in tobacco and maize plants.[11] Protein synthesis in yeast was found to be highly affect by composition of the Kozak sequence in yeast, with adenine enrichment resulting in higher levels of gene expression.[12] A suboptimal Kozak sequence can allow for PIC to scan past the first AUG site and start initiation at a downstream AUG codon.[13][2]
Ribosome assembly
The ribosome assembles on the start codon (AUG), located within the Kozak sequence. Prior to translation initiation, scanning is done by the pre-initiation complex (PIC). The PIC consists of the 40S (small ribosomal subunit) bound the to ternary complex, eIF2-GTP-intiatorMet tRNA (TC) to form the 43S ribosome. Assisted by several other initiation factors (eIF1 and eIF1A, eIF5, eIF3, polyA binding protein) it is recruited to the 5′ end of the mRNA. Eukaryotic mRNA is capped with a 7-methylguanosine (m7G) nucleotide which can helps recruit the PIC to the mRNA and initiate scanning. This recruitment to the m7G 5′ cap is support by the inability of eukaryotic ribosomes to translate circular mRNA, which has no 5′ end.[14] Once the PIC binds to the mRNA it scans until it reaches the first AUG codon in a Kozak sequence.[15][16] This scanning is referred to as the scanning mechanism of initiation.
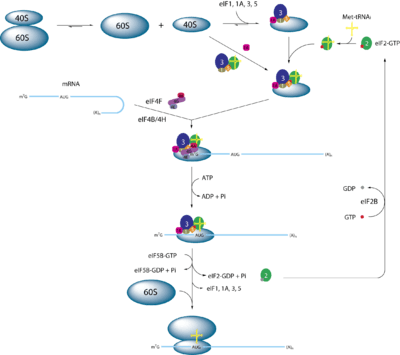
The scanning mechanism of Initiation starts when the PIC binds the 5′ end of the mRNA. Scanning is stimulated by Dhx29 and Ddx3/Ded1 and eIF4 proteins.[1] The Dhx29 and Ddx3/Ded1 are DEAD-box helicases that help to unwind any secondary mRNA structure which could hinder scanning.[17] The scanning of an mRNA continues until the first AUG codon on the mRNA is reached, this is known as the "First AUG Rule".[1] While exceptions to the "First AUG Rule" exist, most exceptions take place at a second AUG codon that is located 3 to 5 nucleotides downstream from the first AUG, or within 10 nucleotides from the 5′ end of the mRNA.[18] At the AUG codon a Methionine tRNA anticodon is recognized by mRNA codon.[19] Upon base pairing to the start codon the eIF5 in the PIC helps to hydrolyze a guanosine triphosphate (GTP) bound to the eIF2.[20][21] This leads to the a structural rearrangement that commits the PIC to binding to the large ribosomal subunit (60S) and forming the ribosomal complex (80S). Once the 80S ribosome complex is formed then the elongation phase of translation starts.
The first start codon closest to the 5′ end of the strand is not always recognized if it is not contained in a Kozak-like sequence. Lmx1b is an example of a gene with a weak Kozak consensus sequence.[22] For initiation of translation from such a site, other features are required in the mRNA sequence in order for the ribosome to recognize the initiation codon. Exceptions to the first AUG rule may occur if it is not contained in a Kozak-like sequence. This is called leaky scanning and could be a potential way to control translation through initiation.[23] For initiation of translation from such a site, other features are required in the mRNA sequence in order for the ribosome to recognize the initiation codon.
It is believed that the PIC is stalled at the Kozak sequence by interactions between eIF2 and the −3 and +4 nucleotides in the Kozak position.[24] This stalling allows the start codon and the corresponding anticodon time to form the correct hydrogen bonding. The Kozak consensus sequence is so common that the similarity of the sequence around the AUG codon to the Kozak Sequence is used as a criterion for finding start codons in eukaryotes.[25]
Differences between eukaryotic and prokaryotic initiation
The scanning mechanism of initiation, which utilizes the Kozak sequence, is found only in eukaryotic organism and has significant differences from the way prokaryotes and archaea initiate translation. The biggest difference is the existence of the Shine-Dalgarno (SD) sequence in mRNA for prokaryotes. The SD sequence is located near the start codon which is in contrast to the Kozak sequence which actually contains the start codon. The Shine Dalgarno sequence allows the 16S subunit of the small ribosome subunit to bind to the AUG start codon immediately with no need for scanning along the mRNA. This results in a more rigorous selection process for the AUG codon than in prokaryotes.[26] An example of prokaryotic start codon promiscuity can be seen in the uses alternate start codons UUG and GUG for some genes.[27]
Mutations and disease
Marilyn Kozak demonstrated, through systematic study of point mutations, that any mutations to a strong consensus sequence in the −3 position or to the +4 position resulted in highly impaired translation initiation both in vitro and in vivo.[28][29]
Research has shown that a mutation of G—>C in the −6 position of the β-globin gene (β+45; human) disrupted the haematological and biosynthetic phenotype function. This was the first mutation found in the Kozak sequence and showed a 30% decrease in translational efficiency. It was found in a family from the Southeast Italy and they suffered from thalassaemia intermedia.[4]
Similar observations were made regarding mutations in the position −5 from the start codon, AUG. Cytosine in this position, as opposed to thymine, showed more efficient translation and increased expression of the platelet adhesion receptor, glycoprotein Ibα in humans.[30]
Mutations to the Kozak sequence can also have drastic effects upon human health, in particular the heart disease with the GATA4 gene. The GATA4 gene is responsible for gene expression in a wide variety of tissues including the heart.[31] When the guanosine at the -6 position in the Kozak sequence of GATA4 is mutated to a cytosine a reduction in GATA4 protein levels, which leads atrial septal defect in the heart.[32]
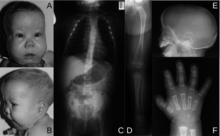
The ability of the Kozak sequence to start translation can result in novel initiation codons in the typically untranslated region of the 5′ (5′ UTR) end of the mRNA transcript. When a G to A mutation was observed in this region it resulted in an out of frame and thus protein mutation. This mutated protein results in campomelic dysplasia. Campomelic dysplasia is a developmental disorder that results in skeletal malformations.[33]
Variations in the consensus sequence
The Kozak consensus has been variously described as:[34]
65432-+234
(gcc)gccRccAUGG (Kozak 1987)
AGNNAUGN
ANNAUGG
ACCAUGG (Spotts et al., 1997, mentioned in Kozak 2002)
GACACCAUGG (H. sapiens HBB, HBD, R. norvegicus Hbb, etc.)
| Biota | Phylum | Consensus sequences |
|---|---|---|
| Vertebrate (Kozak 1987) | gccRccATGG[7] | |
| Fruit fly (Drosophila spp.) | Arthropoda | atMAAMATGamc[35] |
| Budding yeast (Saccharomyces cerevisiae) | Ascomycota | aAaAaAATGTCt[36] |
| Slime mold (Dictyostelium discoideum) | Amoebozoa | aaaAAAATGRna[37] |
| Ciliate | Ciliophora | nTaAAAATGRct[37] |
| Malarial protozoa (Plasmodium spp.) | Apicomplexa | taaAAAATGAan[37] |
| Toxoplasma (Toxoplasma gondii) | Apicomplexa | gncAaaATGg[38] |
| Trypanosomatidae | Euglenozoa | nnnAnnATGnC[37] |
| Terrestrial plants | acAACAATGGC[39] | |
| Microalga (Dunaliella salina) | Chlorophyta | gccaagATGgcg[40] |
See also
- mRNA, the nucleic acid messenger that serves as the middleman in the Central Dogma of Biology
- Ribosome, the molecular machine responsible for protein synthesis
- Shine-Dalgarno sequence, the ribosomal binding site of prokaryotes.
- Translation, the process of peptide synthesis
References
- Kozak, M. (February 1989). "The scanning model for translation: an update". The Journal of Cell Biology. 108 (2): 229–241. doi:10.1083/jcb.108.2.229. ISSN 0021-9525. PMC 2115416. PMID 2645293.
- Kozak, Marilyn (2002-10-16). "Pushing the limits of the scanning mechanism for initiation of translation". Gene. 299 (1): 1–34. doi:10.1016/S0378-1119(02)01056-9. ISSN 0378-1119. PMC 7126118. PMID 12459250.
- Kozak, Marilyn (1999-07-08). "Initiation of translation in prokaryotes and eukaryotes". Gene. 234 (2): 187–208. doi:10.1016/S0378-1119(99)00210-3. ISSN 0378-1119. PMID 10395892.
- De Angioletti M, Lacerra G, Sabato V, Carestia C (2004). "Beta+45 G --> C: a novel silent beta-thalassaemia mutation, the first in the Kozak sequence". Br J Haematol. 124 (2): 224–31. doi:10.1046/j.1365-2141.2003.04754.x. PMID 14687034.
- Hernández, Greco; Osnaya, Vincent G.; Pérez-Martínez, Xochitl (2019-07-25). "Conservation and Variability of the AUG Initiation Codon Context in Eukaryotes". Trends in Biochemical Sciences. 44 (12): 1009–1021. doi:10.1016/j.tibs.2019.07.001. ISSN 0968-0004. PMID 31353284.
- Kozak, M (1984-01-25). "Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs". Nucleic Acids Research. 12 (2): 857–872. doi:10.1093/nar/12.2.857. ISSN 0305-1048. PMC 318541. PMID 6694911.
- Kozak M (October 1987). "An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs". Nucleic Acids Res. 15 (20): 8125–8148. doi:10.1093/nar/15.20.8125. PMC 306349. PMID 3313277.
- Nomenclature for Incompletely Specified Bases in Nucleic Acid Sequences, NC-IUB, 1984.
- Kozak M (1984). "Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo". Nature. 308 (5956): 241–246. Bibcode:1984Natur.308..241K. doi:10.1038/308241a0. PMID 6700727.
- Kozak M (1986). "Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes". Cell. 44 (2): 283–92. doi:10.1016/0092-8674(86)90762-2. PMID 3943125.
- Lukaszewicz, Marcin; Feuermann, Marc; Jérouville, Bénédicte; Stas, Arnaud; Boutry, Marc (2000-05-15). "In vivo evaluation of the context sequence of the translation initiation codon in plants". Plant Science. 154 (1): 89–98. doi:10.1016/S0168-9452(00)00195-3. ISSN 0168-9452. PMID 10725562.
- Li, Jing; Liang, Qiang; Song, Wenjiang; Marchisio, Mario Andrea (2017). "Nucleotides upstream of the Kozak sequence strongly influence gene expression in the yeast S. cerevisiae". Journal of Biological Engineering. 11: 25. doi:10.1186/s13036-017-0068-1. ISSN 1754-1611. PMC 5563945. PMID 28835771.
- Kochetov, Alex V. (2005-04-01). "AUG codons at the beginning of protein coding sequences are frequent in eukaryotic mRNAs with a suboptimal start codon context". Bioinformatics. 21 (7): 837–840. doi:10.1093/bioinformatics/bti136. ISSN 1367-4803. PMID 15531618.
- Kozak, Marilyn (July 1979). "Inability of circular mRNA to attach to eukaryotic ribosomes". Nature. 280 (5717): 82–85. Bibcode:1979Natur.280...82K. doi:10.1038/280082a0. ISSN 1476-4687. PMID 15305588.
- Schmitt, Emmanuelle; Coureux, Pierre-Damien; Monestier, Auriane; Dubiez, Etienne; Mechulam, Yves (2019-02-21). "Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core". International Journal of Molecular Sciences. 20 (4): 939. doi:10.3390/ijms20040939. ISSN 1422-0067. PMC 6412873. PMID 30795538.
- Grzegorski, Steven J.; Chiari, Estelle F.; Robbins, Amy; Kish, Phillip E.; Kahana, Alon (2014). "Natural Variability of Kozak Sequences Correlates with Function in a Zebrafish Model". PLOS One. 9 (9): e108475. Bibcode:2014PLoSO...9j8475G. doi:10.1371/journal.pone.0108475. PMC 4172775. PMID 25248153.
- Hinnebusch, Alan G. (2014). "The Scanning Mechanism of Eukaryotic Translation Initiation". Annual Review of Biochemistry. 83 (1): 779–812. doi:10.1146/annurev-biochem-060713-035802. PMID 24499181.
- Kozak, M. (1995-03-28). "Adherence to the first-AUG rule when a second AUG codon follows closely upon the first". Proceedings of the National Academy of Sciences. 92 (7): 2662–2666. Bibcode:1995PNAS...92.2662K. doi:10.1073/pnas.92.7.2662. ISSN 0027-8424. PMC 42278. PMID 7708701.
- Cigan, A. M.; Feng, L.; Donahue, T. F. (1988-10-07). "tRNAi(met) functions in directing the scanning ribosome to the start site of translation". Science. 242 (4875): 93–97. Bibcode:1988Sci...242...93C. doi:10.1126/science.3051379. ISSN 0036-8075. PMID 3051379.
- Pestova, Tatyana V.; Lomakin, Ivan B.; Lee, Joon H.; Choi, Sang Ki; Dever, Thomas E.; Hellen, Christopher U. T. (January 2000). "The joining of ribosomal subunits in eukaryotes requires eIF5B". Nature. 403 (6767): 332–335. Bibcode:2000Natur.403..332P. doi:10.1038/35002118. ISSN 1476-4687. PMID 10659855.
- Algire, Mikkel A.; Maag, David; Lorsch, Jon R. (2005-10-28). "Pi Release from eIF2, Not GTP Hydrolysis, Is the Step Controlled by Start-Site Selection during Eukaryotic Translation Initiation". Molecular Cell. 20 (2): 251–262. doi:10.1016/j.molcel.2005.09.008. ISSN 1097-2765. PMID 16246727.
- Dunston JA, Hamlington JD, Zaveri J, et al. (September 2004). "The human LMX1B gene: transcription unit, promoter, and pathogenic mutations". Genomics. 84 (3): 565–76. doi:10.1016/j.ygeno.2004.06.002. PMID 15498463.
- Alekhina, O. M.; Vassilenko, K. S. (2012). "Translation initiation in eukaryotes: Versatility of the scanning model". Biochemistry (Moscow). 77 (13): 1465–1477. doi:10.1134/s0006297912130056. PMID 23379522.
- Hinnebusch, Alan G. (September 2011). "Molecular Mechanism of Scanning and Start Codon Selection in Eukaryotes". Microbiology and Molecular Biology Reviews. 75 (3): 434–467. doi:10.1128/MMBR.00008-11. ISSN 1092-2172. PMC 3165540. PMID 21885680.
- Louis, B. G.; Ganoza, M. C. (1988). "Signals determining translational start-site recognition in eukaryotes and their role in prediction of genetic reading frames". Molecular Biology Reports. 13 (2): 103–115. doi:10.1007/bf00539058. ISSN 0301-4851. PMID 3221841.
- Huang, Han-kuei; Yoon, Heejeong; Hannig, Ernest M.; Donahue, Thomas F. (1997-09-15). "GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae". Genes & Development. 11 (18): 2396–2413. doi:10.1101/gad.11.18.2396. ISSN 0890-9369. PMC 316512. PMID 9308967.
- Gualerzi, C. O.; Pon, C. L. (1990-06-26). "Initiation of mRNA translation in prokaryotes". Biochemistry. 29 (25): 5881–5889. doi:10.1021/bi00477a001. ISSN 0006-2960. PMID 2200518.
- Kozak, Marilyn (1986-01-31). "Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes". Cell. 44 (2): 283–292. doi:10.1016/0092-8674(86)90762-2. ISSN 0092-8674. PMID 3943125.
- Kozak, Marilyn (March 1984). "Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo". Nature. 308 (5956): 241–246. Bibcode:1984Natur.308..241K. doi:10.1038/308241a0. ISSN 1476-4687. PMID 6700727.
- Afshar-Kharghan, Vahid; Li, Chester Q.; Khoshnevis-Asl, Mohammad; LóPez, José A. (1999). "Kozak Sequence Polymorphism of the Glycoprotein (GP) Ib Gene is a Major Determinant of the Plasma Membrane Levels of the Platelet GP Ib-IX-V Complex". Blood. 94: 186–191. doi:10.1182/blood.v94.1.186.413k19_186_191.
- Lee, Y.; Shioi, T.; Kasahara, H.; Jobe, S. M.; Wiese, R. J.; Markham, B. E.; Izumo, S. (June 1998). "The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression". Molecular and Cellular Biology. 18 (6): 3120–3129. doi:10.1128/mcb.18.6.3120. ISSN 0270-7306. PMC 108894. PMID 9584153.
- Mohan, Rajiv A.; Engelen, Klaartje van; Stefanovic, Sonia; Barnett, Phil; Ilgun, Aho; Baars, Marieke J. H.; Bouma, Berto J.; Mulder, Barbara J. M.; Christoffels, Vincent M.; Postma, Alex V. (2014). "A mutation in the Kozak sequence of GATA4 hampers translation in a family with atrial septal defects". American Journal of Medical Genetics Part A. 164 (11): 2732–2738. doi:10.1002/ajmg.a.36703. ISSN 1552-4833. PMID 25099673.
- Bohlen, Anna E. von; Böhm, Johann; Pop, Ramona; Johnson, Diana S.; Tolmie, John; Stücker, Ralf; Morris‐Rosendahl, Deborah; Scherer, Gerd (2017). "A mutation creating an upstream initiation codon in the SOX9 5′ UTR causes acampomelic campomelic dysplasia". Molecular Genetics & Genomic Medicine. 5 (3): 261–268. doi:10.1002/mgg3.282. ISSN 2324-9269. PMC 5441400. PMID 28546996.
- Tang, Sen-Lin; Chang, Bill C.H.; Halgamuge, Saman K. (August 2010). "Gene functionality's influence on the second codon: A large-scale survey of second codon composition in three domains". Genomics. 96 (2): 92–101. doi:10.1016/j.ygeno.2010.04.001. PMID 20417269.
- Cavener DR (February 1987). "Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates". Nucleic Acids Res. 15 (4): 1353–61. doi:10.1093/nar/15.4.1353. PMC 340553. PMID 3822832.
- Hamilton R, Watanabe CK, de Boer HA (April 1987). "Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs". Nucleic Acids Res. 15 (8): 3581–93. doi:10.1093/nar/15.8.3581. PMC 340751. PMID 3554144.
- Yamauchi K (May 1991). "The sequence flanking translational initiation site in protozoa". Nucleic Acids Res. 19 (10): 2715–20. doi:10.1093/nar/19.10.2715. PMC 328191. PMID 2041747.
- Seeber, F. (1997). "Consensus sequence of translational initiation sites from Toxoplasma gondii genes". Parasitology Research. 83 (3): 309–311. doi:10.1007/s004360050254. PMID 9089733.
- Lütcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA (January 1987). "Selection of AUG initiation codons differs in plants and animals". EMBO J. 6 (1): 43–8. doi:10.1002/j.1460-2075.1987.tb04716.x. PMC 553354. PMID 3556162.
- Kadkhodaei, Saeid; Hashemi, Farahnaz S. Golestan; Rezaei, Morvarid Akhavan; Abbasiliasi, Sahar; Shun, Tan Joo; Memari, Hamid R. Rajabi; Moradpour, Mahdi; Ariff, Arbakariya B. (2016-07-05). "Cis/transgene optimization: systematic discovery of some key gene expression elements integrating bioinformatics and computational biology". bioRxiv 10.1101/061945.
Further reading
- Kozak M (November 1990). "Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes". Proceedings of the National Academy of Sciences of the United States of America. 87 (21): 8301–5. Bibcode:1990PNAS...87.8301K. doi:10.1073/pnas.87.21.8301. PMC 54943. PMID 2236042.
- Kozak M (November 1991). "An analysis of vertebrate mRNA sequences: intimations of translational control". The Journal of Cell Biology. 115 (4): 887–903. doi:10.1083/jcb.115.4.887. PMC 2289952. PMID 1955461.
- Kozak M (October 2002). "Pushing the limits of the scanning mechanism for initiation of translation". Gene. 299 (1–2): 1–34. doi:10.1016/S0378-1119(02)01056-9. PMC 7126118. PMID 12459250.