SUI1
In molecular biology, the single-domain protein SUI1 is a translation initiation factor often found in the fungus, Saccharomyces cerevisiae (Baker's yeast) but it is also found in other eukaryotes and prokaryotes as well as archaea. It is otherwise known as Eukaryotic translation initiation factor 1 (eIF1) in eukaryotes or YciH in bacteria.[1]
| SUI1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
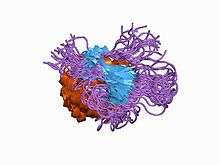 NMR solution structure of E. coli yciH gene. | |||||||||
| Identifiers | |||||||||
| Symbol | SUI1 | ||||||||
| Pfam | PF01253 | ||||||||
| InterPro | IPR001950 | ||||||||
| PROSITE | PDOC00862 | ||||||||
| SCOPe | 2if1 / SUPFAM | ||||||||
| |||||||||
Function
SUI1 is a translation initiation factor that directs the ribosome to the translation start site, helped by eIF2 and the initiator Met-tRNAiMet.[2] SUI1 ensures that translation initiation commences from the correct start codon (usually AUG), by stabilizing the pre-initiation complex around the start codon. SUI1 promotes a high initiation fidelity for the AUG codon, discriminating against non-AUG codons.[3]
In E. coli however, it seems that the SUI1 homolog YciH is an inhibitor of translation during stress instead.[4]
Structure
The primary structure of the SUI1 protein is made up of 108 amino acids. The protein domain has a structure made of a seven-bladed beta-propeller and it also contains a C-terminal alpha helix.[5] Homologues of SUI1 have been found [6] in mammals, insects and plants. SUI1 is also evolutionary related to proteins from Escherichia coli (yciH), Haemophilus influenzae (HI1225) and Methanococcus vannielii.[5]
References
- Prokaryotic+Initiation+Factor-3 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Yoon HJ, Donahue TF (January 1992). "The suil suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNA(iMet) recognition of the start codon". Molecular and Cellular Biology. 12 (1): 248–60. doi:10.1128/mcb.12.1.248. PMC 364089. PMID 1729602.
- Martin-Marcos P, Cheung YN, Hinnebusch AG (December 2011). "Functional elements in initiation factors 1, 1A, and 2β discriminate against poor AUG context and non-AUG start codons". Molecular and Cellular Biology. 31 (23): 4814–31. doi:10.1128/MCB.05819-11. PMC 3232919. PMID 21930786.
- Osterman, IA; Evfratov, SA; Dzama, MM; Pletnev, PI; Kovalchuk, SI; Butenko, IO; Pobeguts, OV; Golovina, AY; Govorun, VM; Bogdanov, AA; Sergiev, PV; Dontsova, OA (2015). "A bacterial homolog YciH of eukaryotic translation initiation factor eIF1 regulates stress-related gene expression and is unlikely to be involved in translation initiation fidelity". RNA Biology. 12 (9): 966–71. doi:10.1080/15476286.2015.1069464. PMC 4615754. PMID 26177339.
- Herrmannová A, Daujotyte D, Yang JC, Cuchalová L, Gorrec F, Wagner S, Dányi I, Lukavsky PJ, Valásek LS (March 2012). "Structural analysis of an eIF3 subcomplex reveals conserved interactions required for a stable and proper translation pre-initiation complex assembly". Nucleic Acids Research. 40 (5): 2294–311. doi:10.1093/nar/gkr765. PMC 3300007. PMID 22090426.
- Fields C, Adams MD (January 1994). "Expressed sequence tags identify a human isolog of the suil translation initiation factor". Biochemical and Biophysical Research Communications. 198 (1): 288–91. doi:10.1006/bbrc.1994.1040. PMID 7904817.