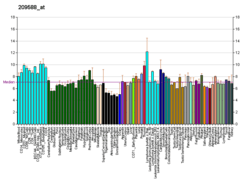
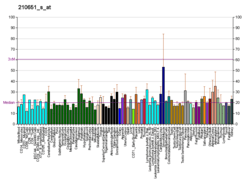
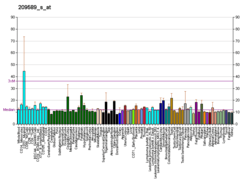
EPH receptor B2
Ephrin type-B receptor 2 is a protein that in humans is encoded by the EPHB2 gene.[5]
Function
Ephrin receptors and their ligands, the ephrins, mediate numerous developmental processes, particularly in the nervous system. Based on their structures and sequence relationships, ephrins are divided into the ephrin-A (EFNA) class, which are anchored to the membrane by a glycosylphosphatidylinositol linkage, and the ephrin-B (EFNB) class, which are transmembrane proteins. The Eph family of receptors are divided into 2 groups based on the similarity of their extracellular domain sequences and their affinities for binding ephrin-A and ephrin-B ligands. Ephrin receptors make up the largest subgroup of the receptor tyrosine kinase (RTK) family. The protein encoded by this gene is a receptor for ephrin-B family members.[6]
Animal studies
EphB2 is part of the NMDA signaling pathway and restoring expression rescues cognitive function in an animal model of Alzheimer's disease.[7]
A recessive EphB2 gene is responsible for the crested-feather mutation in pigeons.[8]
Interactions
EPH receptor B2 has been shown to interact with:
- Abl gene[9]
- RAS p21 protein activator 1[10]
- Src[11][12]
References
- GRCh38: Ensembl release 89: ENSG00000133216 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028664 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Chan J, Watt VM (August 1991). "eek and erk, new members of the eph subclass of receptor protein-tyrosine kinases". Oncogene. 6 (6): 1057–61. PMID 1648701.
- "Entrez Gene: EPHB2 EPH receptor B2".
- Cissé M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, Ho K, Yu GQ, Mucke L (January 2011). "Reversing EphB2 depletion rescues cognitive functions in Alzheimer model". Nature. 469 (7328): 47–52. doi:10.1038/nature09635. PMC 3030448. PMID 21113149. Lay summary – NHS Choices.
- Shapiro MD, Kronenberg Z, Li C, Domyan ET, Pan H, Campbell M, Tan H, Huff CD, Hu H, Vickrey AI, Nielsen SC, Stringham SA, Hu H, Willerslev E, Gilbert MT, Yandell M, Zhang G, Wang J (January 2013). "Genomic diversity and evolution of the head crest in the rock pigeon". Science. 339 (6123): 1063–7. doi:10.1126/science.1230422. PMC 3778192. PMID 23371554. Lay summary – New York Times.
- Yu HH, Zisch AH, Dodelet VC, Pasquale EB (July 2001). "Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor". Oncogene. 20 (30): 3995–4006. doi:10.1038/sj.onc.1204524. PMID 11494128.
- Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T (July 1997). "Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells". EMBO J. 16 (13): 3877–88. doi:10.1093/emboj/16.13.3877. PMC 1170012. PMID 9233798.
- Zisch AH, Kalo MS, Chong LD, Pasquale EB (May 1998). "Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region". Oncogene. 16 (20): 2657–70. doi:10.1038/sj.onc.1201823. PMID 9632142.
- Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB (January 2000). "Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses". Oncogene. 19 (2): 177–87. doi:10.1038/sj.onc.1203304. PMID 10644995.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.