Vandetanib
Vandetanib (INN, trade name Caprelsa)[2] is an anti-cancer drug that is used for the treatment of certain tumours of the thyroid gland. It acts as a kinase inhibitor of a number of cell receptors, mainly the vascular endothelial growth factor receptor (VEGFR), the epidermal growth factor receptor (EGFR), and the RET-tyrosine kinase.[3][4] The drug was developed by AstraZeneca[1] and later on Sanofi.
 | |
| Clinical data | |
|---|---|
| Trade names | Caprelsa |
| Other names | ZD6474 |
| AHFS/Drugs.com | caprelsa |
| MedlinePlus | a611037 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90–96% |
| Metabolism | CYP3A4, FMO1, FMO3 |
| Elimination half-life | 19 days (mean)[1] |
| Excretion | 44% faeces, 25% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.195.611 |
| Chemical and physical data | |
| Formula | C22H24BrFN4O2 |
| Molar mass | 475.362 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medical use
Vandetanib is used to treat medullary thyroid cancer in adults who are ineligible for surgery.[1][5][6]
Contraindications
The V804M mutation in RET confers resistance to Vandetanib anti-RET activity.[6]
In people with moderate and severe hepatic impairment, no dosage for vandetanib has been recommended, as its safety and efficacy has not been established yet.[7] Vandetanib is contraindicated in people with congenital long QT syndrome.[1][4]
Adverse effects
Very common (present in greater than 10% of people) adverse effects include colds, bronchitis, upper respiratory tract infections, urinary tract infections, decreased appetite, low calcium absorption, insomnia, depressed mood, Headache, tingling sensations, weird, painful sensations, dizziness, blurred vision, damage to the cornea, long QT syndrome, high blood pressure, stomach pain, diarrhea, nausea, vomiting, indigestion, sensitivity to sunlight, rash, acne, dry and itchy skin, nail disorders, protein in urine, kidney stones, weakness, fatigue, pain, and edema.[5]
Common (present in between 1% and 10% of people) adverse effects include pneumonia, sepsis, influenza, cystitis, sinusitis, laryngitis, folliculitis, boils, fungal infection, kidney infections, low thyroid hormone levels, low potassium, high calcium levels, hyperglycemia, dehydration, low sodium levels, anxiety, tremor, lethargy, loss of consciousness, balance disorders, changes in sense of taste, visual impairment, halo vision, perceived light flashes, glaucoma, pink eye, dry eye, keratopathy, hypertensive crisis, mini strokes, nose bleeds, coughing up blood, defecating blood, colitis, dry mouth, stomatitis, constipation, gastritis, gallstones, Chemotherapy-induced acral erythema, hair loss, painful urination, bloody urine, kidney failure, frequent urination, urgent need to urinate, and fever.[5]
Interactions
Vandetanib has been reported as a substrate for the OATP1B1 and OATP1B3 transporters. Interaction of vandetanib with OATP1B1 and OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions.[7] Also, vandetanib is an inhibitor of OATP1B3 transporter but not for OATP1B1.[8]
Other drugs that prolong the QT interval can possibly add to this side effect of vandetanib. As the drug is partly metabolised via the liver enzyme CYP3A4, strong inducers of this enzyme can decrease its blood plasma concentrations. CYP3A4 inhibitors do not significantly increase vandetanib concentrations, presumably because it is also metabolised by flavin containing monooxygenase 1 (FMO1) and 3.[1][4]
Pharmacology
Vandetanib is an inhibitor of vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and RET tyrosine kinases. RET tyrosine kinases; it weakly inhibits VEGFR-3.[5][9]
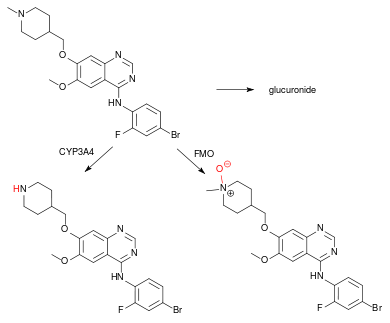
Vandetanib is well absorbed from the gut, reaches peak blood plasma concentrations 4 to 10 hours after application, and has a half-life of 19 days on average, per pharmacokinetic studies. It has to be taken for about three months to achieve a steady-state concentration. In the blood, it is almost completely (90–96%) bound to plasma proteins such as albumin. It is metabolised to N-desmethylvandetanib via CYP3A4 and to vandetanib-N-oxide via FMO1 and 3. Both of these are active metabolites. Vandetanib is excreted via the faeces (44%) and the urine (25%) in form of the unchanged drug and the metabolites.[4][11][10]
History
Vandetanib was approved by the FDA in April 2011 for treatment of late-stage thyroid cancer.[12]
Vandetanib was first initially marketed without a trade name; it has been marketed under the trade name Caprelsa since August 2011.[13]
In 2015 Genzyme acquired the product from AstraZeneca.[14]
Research
AstraZeneca tested Vandetanib in clinical trials for non-small cell lung cancer and submitted an application for approval to the EMA but then withdrew the application in October 2009 after trials showed no benefit when the drug was administered alongside chemotherapy.[15] A clinical trial of vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma was negative in a prospective, randomised, double-blind, multicentre phase 2 trial.[16]
References
- "US Label" (PDF). FDA. July 2016. Index page at FDA website for NDA 022405
- "Vandetanib". AdisInsight. Retrieved 27 February 2017.
- "Definition of vandetanib". NCI Drug Dictionary. National Cancer Institute. 2011-02-02.
- "Vandetanib Monograph". Drugs.com. Retrieved 29 August 2012.
- "UK label". www.medicines.org.uk. UK Electronic Medicines Compendium. 16 December 2016. Retrieved 27 February 2017.
- Viola, D; et al. (April 2016). "Treatment of advanced thyroid cancer with targeted therapies: ten years of experience". Endocrine-Related Cancer. 23 (4): R185–205. doi:10.1530/ERC-15-0555. PMID 27207700.
- Khurana V, Minocha M, Pal D, Mitra AK (March 2014). "Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors". Drug Metabol Drug Interact. 0 (3): 179–90. doi:10.1515/dmdi-2013-0062. PMC 4407685. PMID 24643910.
- Khurana V, Minocha M, Pal D, Mitra AK (May 2014). "Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors". Drug Metabol Drug Interact. 0 (4): 249–59. doi:10.1515/dmdi-2014-0014. PMC 4407688. PMID 24807167.
- Carlomagno, F; Vitagliano, D; Guida, T; Ciardiello, F; Tortora, G; Vecchio, G; Ryan, AJ; Fontanini, G; Fusco, A; Santoro, M (15 December 2002). "ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases". Cancer Research. 62 (24): 7284–90. PMID 12499271.
- "Clinical Pharmacology Review: Vandetanib" (PDF). US Food and Drug Administration, Center for Drug Evaluation and Research. 20 August 2010. Retrieved 29 August 2012.
- Martin, P.; Oliver, S.; Kennedy, S. J.; Partridge, E.; Hutchison, M.; Clarke, D.; Giles, P. (2012). "Pharmacokinetics of Vandetanib: Three Phase I Studies in Healthy Subjects". Clinical Therapeutics. 34 (1): 221–237. doi:10.1016/j.clinthera.2011.11.011. PMID 22206795.
- "FDA approves new treatment for rare form of thyroid cancer". Retrieved 7 April 2011.
- Starkey, Jonathan (August 2, 2011). "AstraZeneca (finally) lands name for cancer drug". Delaware Inc.
- Fourcade, Marthe (27 July 2015). "Sanofi to Buy Caprelsa Drug from AstraZeneca for $300 Million". Bloomberg.
- "Zactima". European Medicines Agency.
- Middleton, Gary; Palmer, Daniel H; Greenhalf, William; Ghaneh, Paula; Jackson, Richard; Cox, Trevor; Evans, Anthony; Shaw, Victoria E; Wadsley, Jonathan; Valle, Juan W; Propper, David; Wasan, Harpreet; Falk, Stephen; Cunningham, David; Coxon, Fareeda; Ross, Paul; Madhusudan, Srinivasan; Wadd, Nick; Corrie, Pippa; Hickish, Tamas; Costello, Eithne; Campbell, Fiona; Rawcliffe, Charlotte; Neoptolemos, John P (2017). "Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial" (PDF). The Lancet Oncology. 18 (4): 486–499. doi:10.1016/S1470-2045(17)30084-0. PMID 28259610.