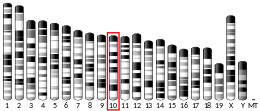
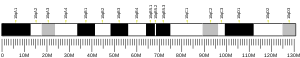
FYN
Proto-oncogene tyrosine-protein kinase Fyn (p59-FYN, Slk, Syn, MGC45350, Gene ID 2534)[5] is an enzyme that in humans is encoded by the FYN gene.[6]
Fyn is a 59-kDa member of the Src family of kinases typically associated with T-cell and neuronal signaling in development and normal cell physiology. Disruptions in these signaling pathways often have implications in the formation of a variety of cancers. By definition as a proto-oncogene, Fyn codes for proteins that help regulate cell growth. Changes in its DNA sequence transform it into an oncogene that leads to the formation of a different protein with implications for normal cell regulation.[5][7]
Fyn is a member of the protein-tyrosine kinase oncogene family. It encodes a membrane-associated tyrosine kinase that has been implicated in the control of cell growth. The protein associates with the p85 subunit of phosphatidylinositol 3-kinase and interacts with the fyn-binding protein. Alternatively spliced transcript variants encoding distinct isoforms exist.[8]
History
Fyn is a member of the Src-family of kinases (SFK), the first proto-oncogene to be identified. The discovery of the Src-family in 1976 led to the Nobel prize for medicine in 1989 for J.M Bishop and E.M. Varmus. Fyn was first identified in 1986 as Syn or Slk through probes derived from v-yes and v-fgr. A common feature of SFKs is that they are commonly upregulated in cancers. Fyn is functionally distinct from its family members in that it interacts with FAK and paxillin (PXN) in the regulation of cell morphology and motility.[9]
Function
Fyn is a protein, present in the signaling pathway of integrins, which activates ras. Fyn is a tyrosine-specific phospho-transferase that is a member of the Src family of non-receptor tyrosine protein kinases.[10] (This family also includes Abl, Src, focal adhesion kinase and Janus kinase.) Fyn is located downstream of several cell surface receptors, commonly associated with neuronal development and T-cell signaling. When fyn is activated it causes downstream activation of molecular signals that drive processes crucial to growth and motility of cells.[9] Fyn is primarily localized to the cytoplasmic leaflet of the plasma membrane, where it phosphorylates tyrosine residues on key targets involved in a variety of different signaling pathways. Tyrosine phosphorylation of target proteins by Fyn serves to either regulate target protein activity, and/or to generate a binding site on the target protein that recruits other signaling molecules. Fyn also is a tumor suppressor. When this normal biology is compromised, the altered Fyn becomes involved in the neoplastic transformation of normal cells to cancerous ones following the pathway from pre-invasive, to invasive, and ultimately metastasis.[7]
Role in signaling pathways
An understanding of the role of fyn in normal biology is crucial to the understanding of its role in cancer, as cancer is the dysregulation of these normal pathways. Knowing which pathways involve Fyn will provide key insight for the development of potential pharmacologic agents to attenuate this uncontrolled signaling.
At least three tools have been useful in discerning a requirement for Fyn function in a particular signaling system:
- cells derived from Fyn-/- mice (as well as cells derived from Fyn, Src, Yes, Fyn triple knockout mice (SYF));
- a kinase-inactive, dominant negative mutant form of Fyn (K299M);
- pharmacologic inhibitors of Src family kinases, such as PP2; note that PP2 also inhibits other tyrosine protein kinases such as Abl, PDGFR and c-Kit.
Using these tools, a requirement for Fyn has been shown for the following signaling pathways: T and B cell receptor signaling,[11][12] integrin-mediated signaling, growth factor and cytokine receptor signaling, platelet activation, ion channel function, cell adhesion, axon guidance, fertilization, entry into mitosis, and differentiation of natural killer cells, oligodendrocytes and keratinocytes. Fyn also has an important role to play in TLR-mediated immune responses from T cells.[13]
Interactions
FYN has been shown to interact with:
- ADD2,[14]
- BCAR1,[15][16]
- C-Raf,[17]
- CBLC,[18]
- CD36,[19][20]
- CD44,[21]
- CDH1,[22]
- CHRNA7,[23]
- CTNND1,[22][24]
- CBL,[25][26]
- CSF1R,[27]
- DLG4,[28][29]
- Dystroglycan,[30]
- EPHA8,[31]
- FYB,[32][33]
- FASLG,[34][35]
- GNB2L1,[36][37]
- GRIN2A,[28][29][38][39]
- ITK,[40][41]
- Janus kinase 2,[42]
- KHDRBS1,[43][44]
- Lck,[45]
- LKB1,[46]
- Nephrin,[47][48]
- PAG1,[49]
- PIK3R2,[50]
- PRKCQ,[51]
- PTK2B,[52][53][54]
- PTK2,[55][56]
- PTPRT[57]
- UNC119,[58]
- RICS,[59]
- SH2D1A,[60][61]
- SKAP1,[33][62][63]
- Syk,[26]
- TNK2,[64]
- TRPC6,[65]
- Tau protein,[66]
- TrkB,[67]
- TYK2,[68]
- TUBA3C,[66]
- WAS,[69][70][71] and
- ZAP-70,[72]
Role in cancer biology
The Src family of kinases is commonly associated with its role in “invasion and tumor progression, epithelial-to-mesenchymal transition, angiogenesis, and development of metastasis,” all hallmarks of cancer progression.[9] Fyn’s normal function in cellular growth and proliferation has the potential to be exploited in the progression and metastasis of cancer cells. Overexpression of Fyn has been found to drive morphologic transformation in normal cells and increase “anchorage-independent growth and prominent morphologic changes.” [5]
Fyn overexpression has been studied in relation to the following cancers: prostate cancer, glioblastoma multiform, squamous cell carcinoma of the head and neck, pancreatic cancer, chronic melogenic leukemia, and melanoma.[5][73] This overexpression triggers a promotion of “anti-apoptotic activity of Akt” in prostate cancer, meaning that these cells have gained the ability to avoid the normal cell death pathways (a common hallmark of cancer).[7] Additionally, in glioblastoma multiform, Src and Fyn have been found to be “effectors of oncogenic EGFR signaling” which has led to tumor invasion and cancer cell survival.[5]
Fyn’s normal role in cell migration and adhesion enables it to utilize the normal cell biology of integrin and FAK for cancer growth. Normal integrin is a cell surface receptor that interacts with the extracellular matrix to send signals influencing cell shape and motility. Normal FAK is a tyrosine kinase that gets recruited to focal adhesion sites and plays a key role in directed cell movement. These normal pathways plan a key role in “mediation of Fyn transmitted cellular events impacting shape and motility.” A compromised version of this pathway would enable cancer cells to change shape and motility, increasing the possibility for advanced invasion and metastasis. Additional pathways under investigation regarding Fyn’s role in cancer progression include: the Rac and Rho family of GTPases, Ras, Erk, and MAPK.[5][7]
Because of this, Fyn has been a common target for anti-cancer therapeutic research. The inhibition of Fyn (like other SFKs) results in decreased cell growth. Furthermore, “expression of kinase-dead-Fyn (KD-Fyn), a specific competitor of endogenous Fyn,” was found to reduce the size of primary tumors in mice. Specifically targeting the unique identifying properties of Fyn as well as inhibiting FAK and PXN has the potential to create a very effective molecularly targeted combination cancer therapy.[7][9] Fyn inhibitors are also being explored as potential therapies for Alzheimer's Disease.[74]
References
- GRCh38: Ensembl release 89: ENSG00000010810 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000019843 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Saito, Yoshihito D.; Jensen, Ana R.; Salgia, Ravi; Posadas, Edwin M. (2010-04-01). "Fyn". Cancer. 116 (7): 1629–1637. doi:10.1002/cncr.24879. ISSN 1097-0142. PMC 2847065. PMID 20151426.
- Semba K, Nishizawa M, Miyajima N, Yoshida MC, Sukegawa J, Yamanashi Y, Sasaki M, Yamamoto T, Toyoshima K (August 1986). "yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family". Proceedings of the National Academy of Sciences of the United States of America. 83 (15): 5459–63. doi:10.1073/pnas.83.15.5459. PMC 386306. PMID 3526330.
- Posadas, Edwin M.; Al-Ahmadie, Hikmat; Robinson, Victoria L.; Jagadeeswaran, Ramasamy; Otto, Kristen; Kasza, Kristen E.; Tretiakov, Maria; Siddiqui, Javed; Pienta, Kenneth J. (2009-01-01). "FYN is overexpressed in human prostate cancer". BJU International. 103 (2): 171–177. doi:10.1111/j.1464-410X.2008.08009.x. ISSN 1464-410X. PMC 2741693. PMID 18990162.
- "Entrez Gene: FYN FYN oncogene related to SRC, FGR, YES".
- Sen, Banibrata; Johnson, Faye M. (2011-04-04). "Regulation of Src Family Kinases in Human Cancers". Journal of Signal Transduction. 2011: 865819. doi:10.1155/2011/865819. ISSN 2090-1739. PMC 3135246. PMID 21776389.
- Resh MD (November 1998). "Fyn, a Src family tyrosine kinase". The International Journal of Biochemistry & Cell Biology. 30 (11): 1159–62. doi:10.1016/S1357-2725(98)00089-2. PMID 9839441.
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B (February 2003). "The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation". Immunological Reviews. 191 (1): 107–18. doi:10.1034/j.1600-065X.2003.00015.x. PMID 12614355.
- Palacios EH, Weiss A (October 2004). "Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation". Oncogene. 23 (48): 7990–8000. doi:10.1038/sj.onc.1208074. PMID 15489916.
- Sharma N, Akhade AS, Qadri A (April 2016). "Src kinases central to T-cell receptor signaling regulate TLR-activated innate immune responses from human T cells". Innate Immunity. 22 (3): 238–244. doi:10.1177/1753425916632305. PMID 26888964.
- Shima T, Okumura N, Takao T, Satomi Y, Yagi T, Okada M, Nagai K (November 2001). "Interaction of the SH2 domain of Fyn with a cytoskeletal protein, beta-adducin". J. Biol. Chem. 276 (45): 42233–40. doi:10.1074/jbc.M102699200. PMID 11526103.
- Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK (April 2000). "Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells". Exp. Cell Res. 256 (1): 168–78. doi:10.1006/excr.2000.4822. PMID 10739664.
- Manié SN, Astier A, Haghayeghi N, Canty T, Druker BJ, Hirai H, Freedman AS (June 1997). "Regulation of integrin-mediated p130(Cas) tyrosine phosphorylation in human B cells. A role for p59(Fyn) and SHP2". J. Biol. Chem. 272 (25): 15636–41. doi:10.1074/jbc.272.25.15636. PMID 9188452.
- Cleghon V, Morrison DK (July 1994). "Raf-1 interacts with Fyn and Src in a non-phosphotyrosine-dependent manner". J. Biol. Chem. 269 (26): 17749–55. PMID 7517401.
- Kim M, Tezuka T, Suziki Y, Sugano S, Hirai M, Yamamoto T (October 1999). "Molecular cloning and characterization of a novel cbl-family gene, cbl-c". Gene. 239 (1): 145–54. doi:10.1016/s0378-1119(99)00356-x. PMID 10571044.
- Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS (September 1991). "Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets". Proc. Natl. Acad. Sci. U.S.A. 88 (17): 7844–8. doi:10.1073/pnas.88.17.7844. PMC 52400. PMID 1715582.
- Bull HA, Brickell PM, Dowd PM (August 1994). "Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells". FEBS Lett. 351 (1): 41–4. doi:10.1016/0014-5793(94)00814-0. PMID 7521304.
- Ilangumaran S, Briol A, Hoessli DC (May 1998). "CD44 selectively associates with active Src family protein tyrosine kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes". Blood. 91 (10): 3901–8. doi:10.1182/blood.V91.10.3901. PMID 9573028.
- Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, Duñach M (April 2003). "p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction". Mol. Cell. Biol. 23 (7): 2287–97. doi:10.1128/mcb.23.7.2287-2297.2003. PMC 150740. PMID 12640114.
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A (April 2001). "alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity". J. Biol. Chem. 276 (17): 13541–6. doi:10.1074/jbc.M008035200. PMID 11278378.
- Martinez MC, Ochiishi T, Majewski M, Kosik KS (July 2003). "Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho". J. Cell Biol. 162 (1): 99–111. doi:10.1083/jcb.200211025. PMC 2172717. PMID 12835311.
- Taher TE, Tjin EP, Beuling EA, Borst J, Spaargaren M, Pals ST (October 2002). "c-Cbl is involved in Met signaling in B cells and mediates hepatocyte growth factor-induced receptor ubiquitination". J. Immunol. 169 (7): 3793–800. doi:10.4049/jimmunol.169.7.3793. PMID 12244174.
- Deckert M, Elly C, Altman A, Liu YC (April 1998). "Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases". J. Biol. Chem. 273 (15): 8867–74. doi:10.1074/jbc.273.15.8867. PMID 9535867.
- Courtneidge SA, Dhand R, Pilat D, Twamley GM, Waterfield MD, Roussel MF (March 1993). "Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor". EMBO J. 12 (3): 943–50. doi:10.1002/j.1460-2075.1993.tb05735.x. PMC 413295. PMID 7681396.
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T (January 1999). "PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A". Proc. Natl. Acad. Sci. U.S.A. 96 (2): 435–40. doi:10.1073/pnas.96.2.435. PMC 15154. PMID 9892651.
- Hou XY, Zhang GY, Yan JZ, Chen M, Liu Y (November 2002). "Activation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemia". Brain Res. 955 (1–2): 123–32. doi:10.1016/s0006-8993(02)03376-0. PMID 12419528.
- Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP (December 2001). "Tyrosine phosphorylation of beta-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins". Biochemistry. 40 (48): 14585–92. doi:10.1021/bi011247r. PMID 11724572.
- Choi S, Park S (September 1999). "Phosphorylation at Tyr-838 in the kinase domain of EphA8 modulates Fyn binding to the Tyr-615 site by enhancing tyrosine kinase activity". Oncogene. 18 (39): 5413–22. doi:10.1038/sj.onc.1202917. PMID 10498895.
- da Silva AJ, Janssen O, Rudd CE (December 1993). "T cell receptor zeta/CD3-p59fyn(T)-associated p120/130 binds to the SH2 domain of p59fyn(T)". J. Exp. Med. 178 (6): 2107–13. doi:10.1084/jem.178.6.2107. PMC 2191307. PMID 7504057.
- Marie-Cardine A, Hendricks-Taylor LR, Boerth NJ, Zhao H, Schraven B, Koretzky GA (October 1998). "Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130". J. Biol. Chem. 273 (40): 25789–95. doi:10.1074/jbc.273.40.25789. PMID 9748251.
- Wenzel J, Sanzenbacher R, Ghadimi M, Lewitzky M, Zhou Q, Kaplan DR, Kabelitz D, Feller SM, Janssen O (December 2001). "Multiple interactions of the cytosolic polyproline region of the CD95 ligand: hints for the reverse signal transduction capacity of a death factor". FEBS Lett. 509 (2): 255–62. doi:10.1016/s0014-5793(01)03174-x. PMID 11741599.
- Hane M, Lowin B, Peitsch M, Becker K, Tschopp J (October 1995). "Interaction of peptides derived from the Fas ligand with the Fyn-SH3 domain". FEBS Lett. 373 (3): 265–8. doi:10.1016/0014-5793(95)01051-f. PMID 7589480.
- Yaka R, He DY, Phamluong K, Ron D (March 2003). "Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1". J. Biol. Chem. 278 (11): 9630–8. doi:10.1074/jbc.M209141200. PMID 12524444.
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D (April 2002). "NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1". Proc. Natl. Acad. Sci. U.S.A. 99 (8): 5710–5. doi:10.1073/pnas.062046299. PMC 122836. PMID 11943848.
- Ma J, Zhang GY (September 2003). "Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia". Neurosci. Lett. 348 (3): 185–9. doi:10.1016/s0304-3940(03)00784-5. PMID 12932824.
- Takagi N, Cheung HH, Bissoon N, Teves L, Wallace MC, Gurd JW (August 1999). "The effect of transient global ischemia on the interaction of Src and Fyn with the N-methyl-D-aspartate receptor and postsynaptic densities: possible involvement of Src homology 2 domains". J. Cereb. Blood Flow Metab. 19 (8): 880–8. doi:10.1097/00004647-199908000-00007. PMID 10458595.
- Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ (January 2000). "Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade". J. Biol. Chem. 275 (3): 2219–30. doi:10.1074/jbc.275.3.2219. PMID 10636929.
- Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ (October 1996). "Identification of Itk/Tsk Src homology 3 domain ligands". J. Biol. Chem. 271 (41): 25646–56. doi:10.1074/jbc.271.41.25646. PMID 8810341.
- Sayeski PP, Ali MS, Safavi A, Lyles M, Kim SO, Frank SJ, Bernstein KE (November 1999). "A catalytically active Jak2 is required for the angiotensin II-dependent activation of Fyn". J. Biol. Chem. 274 (46): 33131–42. doi:10.1074/jbc.274.46.33131. PMID 10551884.
- Oneyama C, Nakano H, Sharma SV (March 2002). "UCS15A, a novel small molecule, SH3 domain-mediated protein-protein interaction blocking drug". Oncogene. 21 (13): 2037–50. doi:10.1038/sj.onc.1205271. PMID 11960376.
- Fusaki N, Iwamatsu A, Iwashima M, Fujisawa Ji (March 1997). "Interaction between Sam68 and Src family tyrosine kinases, Fyn and Lck, in T cell receptor signaling". J. Biol. Chem. 272 (10): 6214–9. doi:10.1074/jbc.272.10.6214. PMID 9045636.
- Filipp D, Moemeni B, Ferzoco A, Kathirkamathamby K, Zhang J, Ballek O, Davidson D, Veillette A, Julius M (2008). "Lck-dependent Fyn activation requires C terminus-dependent targeting of kinase-active Lck to lipid rafts". The Journal of Biological Chemistry. 283 (39): 26409–22. doi:10.1074/jbc.M710372200. PMC 3258908. PMID 18660530.
- Yamada E, Bastie CC (2014). "Disruption of Fyn SH3 domain interaction with a proline-rich motif in liver kinase B1 results in activation of AMP-activated protein kinase". PLoS ONE. 9 (2): e89604. doi:10.1371/journal.pone.0089604. PMC 3934923. PMID 24586906.
- Lahdenperä J, Kilpeläinen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K (August 2003). "Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases". Kidney Int. 64 (2): 404–13. doi:10.1046/j.1523-1755.2003.00097.x. PMID 12846735.
- Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB (June 2003). "Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin". J. Biol. Chem. 278 (23): 20716–23. doi:10.1074/jbc.M301689200. PMID 12668668.
- Brdicka T, Pavlistová D, Leo A, Bruyns E, Korínek V, Angelisová P, Scherer J, Shevchenko A, Hilgert I, Cerný J, Drbal K, Kuramitsu Y, Kornacker B, Horejsí V, Schraven B (May 2000). "Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation". J. Exp. Med. 191 (9): 1591–604. doi:10.1084/jem.191.9.1591. PMC 2213442. PMID 10790433.
- Gout I, Dhand R, Panayotou G, Fry MJ, Hiles I, Otsu M, Waterfield MD (December 1992). "Expression and characterization of the p85 subunit of the phosphatidylinositol 3-kinase complex and a related p85 beta protein by using the baculovirus expression system". Biochem. J. 288. 288 (2): 395–405. doi:10.1042/bj2880395. PMC 1132024. PMID 1334406.
- Ron D, Napolitano EW, Voronova A, Vasquez NJ, Roberts DN, Calio BL, Caothien RH, Pettiford SM, Wellik S, Mandac JB, Kauvar LM (July 1999). "Direct interaction in T-cells between thetaPKC and the tyrosine kinase p59fyn". J. Biol. Chem. 274 (27): 19003–10. doi:10.1074/jbc.274.27.19003. PMID 10383400.
- Ganju RK, Hatch WC, Avraham H, Ona MA, Druker B, Avraham S, Groopman JE (March 1997). "RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes". J. Exp. Med. 185 (6): 1055–63. doi:10.1084/jem.185.6.1055. PMC 2196239. PMID 9091579.
- Katagiri T, Takahashi T, Sasaki T, Nakamura S, Hattori S (June 2000). "Protein-tyrosine kinase Pyk2 is involved in interleukin-2 production by Jurkat T cells via its tyrosine 402". J. Biol. Chem. 275 (26): 19645–52. doi:10.1074/jbc.M909828199. PMID 10867021.
- Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A (April 1997). "Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling". J. Exp. Med. 185 (7): 1253–9. doi:10.1084/jem.185.7.1253. PMC 2196260. PMID 9104812.
- Messina S, Onofri F, Bongiorno-Borbone L, Giovedì S, Valtorta F, Girault JA, Benfenati F (January 2003). "Specific interactions of neuronal focal adhesion kinase isoforms with Src kinases and amphiphysin". J. Neurochem. 84 (2): 253–65. doi:10.1046/j.1471-4159.2003.01519.x. PMID 12558988.
- Arold ST, Ulmer TS, Mulhern TD, Werner JM, Ladbury JE, Campbell ID, Noble ME (May 2001). "The role of the Src homology 3-Src homology 2 interface in the regulation of Src kinases". J. Biol. Chem. 276 (20): 17199–205. doi:10.1074/jbc.M011185200. PMID 11278857.
- Lim SH, Kwon SK, Lee MK, Moon J, Jeong DG, Park E, Kim SJ, Park BC, Lee SC, Ryu SE, Yu DY, Chung BH, Kim E, Myung PK, Lee JR (2009). "Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn". EMBO J. 28 (22): 3564–78. doi:10.1038/emboj.2009.289. PMC 2782100. PMID 19816407.
- Gorska MM, Stafford SJ, Cen O, Sur S, Alam R (February 2004). "Unc119, a novel activator of Lck/Fyn, is essential for T cell activation". J. Exp. Med. 199 (3): 369–79. doi:10.1084/jem.20030589. PMC 2211793. PMID 14757743.
- Taniguchi S, Liu H, Nakazawa T, Yokoyama K, Tezuka T, Yamamoto T (June 2003). "p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn". Biochem. Biophys. Res. Commun. 306 (1): 151–5. doi:10.1016/s0006-291x(03)00923-9. PMID 12788081.
- Li C, Iosef C, Jia CY, Han VK, Li SS (February 2003). "Dual functional roles for the X-linked lymphoproliferative syndrome gene product SAP/SH2D1A in signaling through the signaling lymphocyte activation molecule (SLAM) family of immune receptors". J. Biol. Chem. 278 (6): 3852–9. doi:10.1074/jbc.M206649200. PMID 12458214.
- Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ (February 2003). "SAP couples Fyn to SLAM immune receptors". Nat. Cell Biol. 5 (2): 155–60. doi:10.1038/ncb920. PMID 12545174.
- Marie-Cardine A, Bruyns E, Eckerskorn C, Kirchgessner H, Meuer SC, Schraven B (June 1997). "Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes". J. Biol. Chem. 272 (26): 16077–80. doi:10.1074/jbc.272.26.16077. PMID 9195899.
- Wu L, Yu Z, Shen SH (October 2002). "SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T cell receptor activation". J. Biol. Chem. 277 (43): 40420–7. doi:10.1074/jbc.M206023200. PMID 12171928.
- Linseman DA, Heidenreich KA, Fisher SK (February 2001). "Stimulation of M3 muscarinic receptors induces phosphorylation of the Cdc42 effector activated Cdc42Hs-associated kinase-1 via a Fyn tyrosine kinase signaling pathway". J. Biol. Chem. 276 (8): 5622–8. doi:10.1074/jbc.M006812200. PMID 11087735.
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K (April 2004). "Regulation of TRPC6 channel activity by tyrosine phosphorylation". J. Biol. Chem. 279 (18): 18887–94. doi:10.1074/jbc.M311274200. PMID 14761972.
- Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J (February 2002). "Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau". J. Neurosci. 22 (3): 698–707. doi:10.1523/JNEUROSCI.22-03-00698.2002. PMC 6758498. PMID 11826099.
- Iwasaki Y, Gay B, Wada K, Koizumi S (July 1998). "Association of the Src family tyrosine kinase Fyn with TrkB". J. Neurochem. 71 (1): 106–11. doi:10.1046/j.1471-4159.1998.71010106.x. PMID 9648856.
- Uddin S, Sher DA, Alsayed Y, Pons S, Colamonici OR, Fish EN, White MF, Platanias LC (June 1997). "Interaction of p59fyn with interferon-activated Jak kinases". Biochem. Biophys. Res. Commun. 235 (1): 83–8. doi:10.1006/bbrc.1997.6741. PMID 9196040.
- Banin S, Truong O, Katz DR, Waterfield MD, Brickell PM, Gout I (August 1996). "Wiskott-Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases". Curr. Biol. 6 (8): 981–8. doi:10.1016/s0960-9822(02)00642-5. PMID 8805332.
- Banin S, Gout I, Brickell P (August 1999). "Interaction between Wiskott-Aldrich Syndrome protein (WASP) and the Fyn protein-tyrosine kinase". Mol. Biol. Rep. 26 (3): 173–7. doi:10.1023/A:1006954206151. PMID 10532312.
- Rivero-Lezcano OM, Marcilla A, Sameshima JH, Robbins KC (October 1995). "Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains". Mol. Cell. Biol. 15 (10): 5725–31. doi:10.1128/MCB.15.10.5725. PMC 230823. PMID 7565724.
- Neumeister EN, Zhu Y, Richard S, Terhorst C, Chan AC, Shaw AS (June 1995). "Binding of ZAP-70 to phosphorylated T-cell receptor zeta and eta enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins". Mol. Cell. Biol. 15 (6): 3171–8. doi:10.1128/mcb.15.6.3171. PMC 230549. PMID 7760813.
- Yadav, Vipin; Denning, Mitchell F. (2011-05-01). "Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration". Molecular Carcinogenesis. 50 (5): 346–352. doi:10.1002/mc.20716. ISSN 1098-2744. PMC 3080437. PMID 21480388.
- Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. (2015). "A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease". Alzheimer's Research & Therapy. 7 (1): 35. doi:10.1186/s13195-015-0119-0. PMC 4396171. PMID 25874001.
Further reading
- O'Sullivan E, Kinnon C, Brickell P (1999). "Wiskott-Aldrich syndrome protein, WASP". Int. J. Biochem. Cell Biol. 31 (3–4): 383–7. doi:10.1016/S1357-2725(98)00118-6. PMID 10224664.
- Sasaoka T, Kobayashi M (2000). "The functional significance of Shc in insulin signaling as a substrate of the insulin receptor". Endocr. J. 47 (4): 373–81. doi:10.1507/endocrj.47.373. PMID 11075717.
- Leavitt SA, SchOn A, Klein JC, Manjappara U, Chaiken IM, Freire E (2004). "Interactions of HIV-1 proteins gp120 and Nef with cellular partners define a novel allosteric paradigm". Curr. Protein Pept. Sci. 5 (1): 1–8. doi:10.2174/1389203043486955. PMID 14965316.
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.