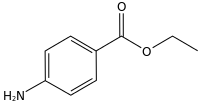
Benzocaine
Benzocaine, sold under the brand name Orajel amongst others, is an ester local anesthetic commonly used as a topical pain reliever or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is also combined with antipyrine to form A/B otic drops to relieve ear pain and remove earwax. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old.[2]. In European countries, the contraindication applies to children under 12 years of age.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anbesol, Cepacol, Lanacane, Orajel, Anesthesin[1] |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.094 |
| Chemical and physical data | |
| Formula | C9H11NO2 |
| Molar mass | 165.192 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was first synthesised in 1890 in Germany and approved for medical use in 1902.[3]
Medical uses
Benzocaine is indicated to treat a variety of pain-related conditions. It may be used for:
- Local anesthesia of oral and pharyngeal mucous membranes (sore throat, cold sores, mouth ulcers, toothache, sore gums, denture irritation)[4]
- Otic pain (earache)[4]
- Surgical or procedural local anesthesia[5]
Other uses

Benzocaine is used as a key ingredient in numerous pharmaceuticals:
- Some glycerol-based ear medications for use in removing excess wax as well as relieving ear conditions such as otitis media and swimmers ear.
- Some previous diet products such as Ayds.
- Some condoms designed to prevent premature ejaculation. Benzocaine largely inhibits sensitivity on the penis, and can allow for an erection to be maintained longer (in a continuous act) by delaying ejaculation. Conversely, an erection will also fade faster if stimulus is interrupted.[6][7]
- Benzocaine mucoadhesive patches have been used in reducing orthodontic pain.[8]
- In Poland it is included, together with menthol and zinc oxide, in the liquid powder (not to be confused with the liquid face powder) used mainly after mosquito bites. Today's ready made Pudroderm[9] was once used there as pharmaceutical compound.
Side effects
Benzocaine is generally well tolerated and non-toxic when applied topically as recommended.[15]
However, there have been reports of serious, life-threatening adverse effects (e.g., seizures, coma, irregular heart beat, respiratory depression) with over-application of topical products or when applying topical products that contain high concentrations of benzocaine to the skin.[16]
Overapplication of oral anesthetics such as benzocaine can increase the risk of pulmonary aspiration by relaxing the gag-reflex and allowing regurgitated stomach contents or oral secretions to enter the airway. Applying an oral anesthetic and consuming beverages before going to bed can be particularly hazardous.
The topical use of higher concentration (10–20%) benzocaine products applied to the mouth or mucous membranes has been found to be a cause of methemoglobinemia, a disorder in which the amount of oxygen carried by the blood is greatly reduced.[17] This side effect is most common in children under two years of age.[18] As a result, the FDA has stated that benzocaine products should not be used in children under two years of age, unless directed by and supervised by a healthcare professional.[19] In European countries, the contraindication applies to children under 12 years of age. Symptoms of methemoglobinemia usually occur within minutes to hours of applying benzocaine, and can occur upon the first-time use or after additional use.[19]
Benzocaine may cause allergic reactions.[20][21][22][23] These include:
- Contact dermatitis (redness and itchiness)[24]
- Anaphylaxis (rare)[24]
Pharmacology
Pharmacodynamics
Pain is caused by the stimulation of free nerve endings. When the nerve endings are stimulated, sodium enters the neuron, causing depolarization of the nerve and subsequent initiation of an action potential. The action potential is propagated down the nerve toward the central nervous system, which interprets this as pain. Benzocaine acts to inhibit the voltage-dependent sodium channels (VDSCs) on the neuron membrane, stopping the propagation of the action potential.
Chemistry
Benzocaine is the ethyl ester of p-aminobenzoic acid (PABA). It can be prepared from PABA and ethanol[25] by Fischer esterification or via the reduction of ethyl p-nitrobenzoate. Benzocaine is sparingly soluble in water; it is more soluble in dilute acids and very soluble in ethanol, chloroform, and ethyl ether. The melting point of benzocaine is 88–90 °C,[26] and the boiling point is about 310 °C.[27] The density of benzocaine is 1.17 g/cm3.
Benzocaine is commonly found, particularly in Britain, as an additive in street cocaine and also as a bulking agent in "legal highs".[28] Whilst giving a numbing effect similar to cocaine, users prefer Benzocaine as it is a better bulking and binding agent that can't be detected once mixed. Benzocaine is the most popular cutting agent worldwide,[29] Benzocaine was used in synthesis of leteprinim. Treatment of benzocaine with hydrazine leads to aminostimil - a compound related to isoniazid.
History
Benzocaine was first synthesized in 1890 by the German chemist Eduard Ritsert (1859–1946),[35] in the town of Eberbach[36] and introduced to the market in 1902 under the name "Anästhesin".[37][38]
Veterinary medicine
A bath solution of benzocaine has been used to anesthetize amphibians for surgery.[39][40]
References
- Pubchem. "Benzocaine". pubchem.ncbi.nlm.nih.gov.
- "Safety Alerts for Human Medical Products - Oral Over-the-Counter Benzocaine Products: Drug Safety Communication - Risk of Serious and Potentially Fatal Blood Disorder". www.fda.gov. Retrieved May 26, 2018.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 475. ISBN 9783527607495.
- AHFS Drug Information 2007. McEvoy GK, ed. Benzocaine. Bethesda, MD: American Society of Health-System Pharmacists; 2007: 2844-5.
- Sultan Healthcare. Topex®metered spray (benzocaine 20%) prescribing information. Englewood, NJ; 2006.
- "'Longer-lasting' condom launched". BBC News. June 17, 2002.
- Garner, Dwight (December 15, 2002). "Endurance Condoms". The New York Times.
- Eslamian L, Borzabadi-Farahani A, Edini HZ, Badiee MR, Lynch E, Mortazavi A (2013). "The analgesic effect of benzocaine mucoadhesive patches on orthodontic pain caused by elastomeric separators, a preliminary study". Acta Odontol Scand. 71 (5): 1168–73. doi:10.3109/00016357.2012.757358. PMID 23301559.
- "Produkty | PUDRODERM" (in Polish). Archived from the original on October 18, 2016. Retrieved May 9, 2014.
- "Cepacol®". Cepacol.com. Retrieved June 2, 2015.
- "Ultra Chloraseptic Anaesthetic Throat Spray 0.71% - Summary of Product Characteristics (SmPC) - (eMC)". www.medicines.org.uk. Retrieved May 5, 2018.
- "Topex® Metered Spray-Sultan Healthcare". Sultanhc.com. Retrieved June 2, 2015.
- "Orajel™ - Oral Care for the Whole Family". Orajel.com. May 11, 2015. Retrieved June 2, 2015.
- "Colgate Orabase Paste with Benzocaine | Indications | Dental Products". Colgateprofessional.com. Retrieved June 2, 2015.
- Lexicomp Online, Adult and Pediatric Lexi-Drugs Online, Hudson, Ohio: Lexi-Comp, Inc.; 2013; April 15, 2013.
- Food and Drug Administration. FDA Public Health Advisory: Life-threatening side effects with use of skin products containing numbing ingredients for cosmetic procedures. 2007 Feb 6, updated 2007 Feb 9. From FDA website
- Shua-Haim, J. R.; Gross, J. S. (1995). "Methemoglobinemia toxicity from topical benzocaine spray". Journal of the American Geriatrics Society. 43 (5): 590. doi:10.1111/j.1532-5415.1995.tb06117.x. PMID 7730550.
- "Benzocaine Topical Products: Sprays, Gels and Liquids – Risk of Methemoglobinemia". Drugs.com. Retrieved March 20, 2014.
- "FDA Drug Safety Communication: Reports of a rare, but serious and potentially fatal adverse effect with the use of over-the-counter (OTC) benzocaine gels and liquids applied to the gums or mouth". Fda.gov. Retrieved June 2, 2015.
- "Allergy to Benzocaine" (PDF). Archived from the original (PDF) on March 20, 2014. Retrieved March 20, 2014.
- Sidhu, S. K.; Shaw, S; Wilkinson, J. D. (1999). "A 10-year retrospective study on benzocaine allergy in the United Kingdom". American Journal of Contact Dermatitis. 10 (2): 57–61. doi:10.1016/s1046-199x(99)90000-3. PMID 10357712.
- González-Rodríguez, A. J.; Gutiérrez-Paredes, E. M.; Revert Fernández, Á.; Jordá-Cuevas, E (2013). "Allergic contact dermatitis to benzocaine: The importance of concomitant positive patch test results" (PDF). Actas Dermo-Sifiliográficas. 104 (2): 156–8. doi:10.1016/j.ad.2011.07.023. PMID 22551703. Archived from the original (PDF) on March 3, 2016. Retrieved March 20, 2014.
- Leslie Goldman (February 5, 2008). "Go easy on medicated lotions, creams, gels". CNN. Retrieved March 20, 2014.
- Cetylite Industries. Cetacaine® (benzocaine 14%, tetracaine 2% and butamben 2%) spray, gel and liquid prescribing information. Pennsauken, NJ; 2006 Sept.
- Demare, Patricia; Regla, Ignacio (2012). "Synthesis of Two Local Anesthetics from Toluene: An Organic Multistep Synthesis in a Project-Oriented Laboratory Course". Journal of Chemical Education. 89: 147. doi:10.1021/ed100838a.
- "Monographs: Pharmaceutical substances: Benzocainum – Benzocaine". The International Pharmacopoeia. Retrieved September 29, 2009.
- D'Ans-Lax, Taschenbuch für Chemiker und Physiker, 4. Auflage, Band 2, Springer Verlag 1982, ISBN 3-540-12263-X
- "Drug war targets cutting agents". August 9, 2010 – via www.bbc.co.uk.
- "Drug prices: All cut up : Cocaine is cheaper, but weaker". The Economist. August 11, 2012.
- Salkowski, H. (1895). "Ueber Esterbildung bei aromatischen Amidosäuren". Berichte der Deutschen Chemischen Gesellschaft. 28 (2): 1917–1923. doi:10.1002/cber.189502802150.
- Erlenmeyer, E.; Wanklyn, J. A. (1865). "Ueber das durch Einwirkung von Jodwasserstoff auf Mannit beziehungsweise auf Melampyrin (Dulcit) entstehende β Hexyljodür und einige seiner Derivate". Annalen der Chemie und Pharmacie. 135 (2): 129. doi:10.1002/jlac.18651350202.
- Limpricht, H. (1898). "Ueber die Verbindungen aus Benzoylchlorid oder Phtalylchlorid und den Estern der drei Oxybenzoësäuren". Justus Liebig's Annalen der Chemie. 303 (3): 274–289. doi:10.1002/jlac.18983030303.
- Adams, Roger; Cohen, F.L. (1928). "Ethyl p-aminobenzoate". Organic Syntheses. 8: 66.; Collective Volume, 1, p. 240
- Ali, S. L. (1983). Benzocaine. Analytical Profiles of Drug Substances. 12. pp. 73–104. doi:10.1016/S0099-5428(08)60164-1. ISBN 978-0-12-260812-4.
- Biography of Eduard Ritsert (in German): Deutsche Biographie: Ritsert, Eduard.
- "100 years of Dr. Ritsert". Dr. Ritsert Pharma. Archived from the original on September 9, 2017. Retrieved March 14, 2010.
- Harry Auterhoff, Lehrbuch der pharmazeutischen Chemie (Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft, 1968).
- Ritsert, E. (1925) "Über den Werdegang des Anästhesins" (On the development of Anästhesin), Pharmazeutische Zeitung, vol. 60, pages 1006–1008. See also: Christoph Friedrich and Magdalena Klimonow, "150. Geburtstag: Eduard Ritsert und das Anaesthesin" Archived March 20, 2014, at the Wayback Machine (150th birthday: Eduard Ritsert and Anästhetsin [Benzocaine]"), Pharmazeutische Zeitung online. First published clinical study demonstrating the efficacy of benzocaine: Noorden, C. v. (1902) "Ueber para-Aminobenzoesäure-Ester als locales Anästhetikum" (On [an] ester of para-aminobenzoic acid as a local anaesthetic), Klinische Wochenschrift, vol. 39, pages 373–375.
- Guénette, SA; Giroux, MC; Vachon, P (2013). "Pain perception and anaesthesia in research frogs". Experimental Animals. 62 (2): 87–92. doi:10.1538/expanim.62.87. PMID 23615302.
- "Amphibian Anesthesia — Research at Penn State". PennState Animal Resource Program. State College, U.S.: The Pennsylvania State University. November 8, 2010. Retrieved August 24, 2017.