Pain
Pain is a distressing feeling often caused by intense or damaging stimuli. The International Association for the Study of Pain's widely used definition defines pain as "An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage".[1] In medical diagnosis, pain is regarded as a symptom of an underlying condition.
| Pain | |
|---|---|
 | |
| A woman grimacing while having blood drawn | |
| Pronunciation |
|
| Specialty | Neurology |
| Duration | typically depends on the cause |
| Types | Physical, Psychological, Psychogenic |
| Medication | Analgesic |
Pain motivates the individual to withdraw from damaging situations, to protect a damaged body part while it heals, and to avoid similar experiences in the future.[2] Most pain resolves once the noxious stimulus is removed and the body has healed, but it may persist despite removal of the stimulus and apparent healing of the body. Sometimes pain arises in the absence of any detectable stimulus, damage or disease.[3]
Pain is the most common reason for physician consultation in most developed countries.[4][5] It is a major symptom in many medical conditions, and can interfere with a person's quality of life and general functioning.[6] Simple pain medications are useful in 20% to 70% of cases.[7] Psychological factors such as social support, hypnotic suggestion, cognitive behavioral therapy, excitement, or distraction can affect pain's intensity or unpleasantness.[8][9] In some debates regarding physician-assisted suicide or euthanasia, pain has been used as an argument to permit people who are terminally ill to end their lives.[10]
Classification
Duration
Pain is usually transitory, lasting only until the noxious stimulus is removed or the underlying damage or pathology has healed, but some painful conditions, such as rheumatoid arthritis, peripheral neuropathy, cancer and idiopathic pain, may persist for years. Pain that lasts a long time is called chronic or persistent, and pain that resolves quickly is called acute. Traditionally, the distinction between acute and chronic pain has relied upon an arbitrary interval of time between onset and resolution; the two most commonly used markers being 3 months and 6 months since the onset of pain,[11] though some theorists and researchers have placed the transition from acute to chronic pain at 12 months.[12]:93 Others apply acute to pain that lasts less than 30 days, chronic to pain of more than six months' duration, and subacute to pain that lasts from one to six months.[13] A popular alternative definition of chronic pain, involving no arbitrarily fixed durations, is "pain that extends beyond the expected period of healing".[11] Chronic pain may be classified as cancer pain or else as benign.[13]
Allodynia
Allodynia is pain experienced in response to a normally painless stimulus.[14] It has no biological function and is classified by stimuli into dynamic mechanical, punctate and static.[14][15] In osteoarthritis, NGF has been identified as being involved in allodynia.[15] The extent and intensity of sensation can be assessed through locating trigger points and the region of sensation, as well as utilising phantom maps.[14]
Phantom
Phantom pain is pain felt in a part of the body that has been amputated, or from which the brain no longer receives signals. It is a type of neuropathic pain.[16]
The prevalence of phantom pain in upper limb amputees is nearly 82%, and in lower limb amputees is 54%.[16] One study found that eight days after amputation, 72% of patients had phantom limb pain, and six months later, 67% reported it.[17][18] Some amputees experience continuous pain that varies in intensity or quality; others experience several bouts of pain per day, or it may reoccur less often. It is often described as shooting, crushing, burning or cramping. If the pain is continuous for a long period, parts of the intact body may become sensitized, so that touching them evokes pain in the phantom limb. Phantom limb pain may accompany urination or defecation.[19]:61–9
Local anesthetic injections into the nerves or sensitive areas of the stump may relieve pain for days, weeks, or sometimes permanently, despite the drug wearing off in a matter of hours; and small injections of hypertonic saline into the soft tissue between vertebrae produces local pain that radiates into the phantom limb for ten minutes or so and may be followed by hours, weeks or even longer of partial or total relief from phantom pain. Vigorous vibration or electrical stimulation of the stump, or current from electrodes surgically implanted onto the spinal cord, all produce relief in some patients.[19]:61–9
Mirror box therapy produces the illusion of movement and touch in a phantom limb which in turn may cause a reduction in pain.[20]
Paraplegia, the loss of sensation and voluntary motor control after serious spinal cord damage, may be accompanied by girdle pain at the level of the spinal cord damage, visceral pain evoked by a filling bladder or bowel, or, in five to ten per cent of paraplegics, phantom body pain in areas of complete sensory loss. This phantom body pain is initially described as burning or tingling but may evolve into severe crushing or pinching pain, or the sensation of fire running down the legs or of a knife twisting in the flesh. Onset may be immediate or may not occur until years after the disabling injury. Surgical treatment rarely provides lasting relief.[19]:61–9
Breakthrough
Breakthrough pain is transitory pain that comes on suddenly and is not alleviated by the patient's regular pain management. It is common in cancer patients who often have background pain that is generally well-controlled by medications, but who also sometimes experience bouts of severe pain that from time to time "breaks through" the medication. The characteristics of breakthrough cancer pain vary from person to person and according to the cause. Management of breakthrough pain can entail intensive use of opioids, including fentanyl.[21][22]
Asymbolia and insensitivity
The ability to experience pain is essential for protection from injury, and recognition of the presence of injury. Episodic analgesia may occur under special circumstances, such as in the excitement of sport or war: a soldier on the battlefield may feel no pain for many hours from a traumatic amputation or other severe injury.[23]
Although unpleasantness is an essential part of the IASP definition of pain,[24] it is possible to induce a state described as intense pain devoid of unpleasantness in some patients, with morphine injection or psychosurgery.[25] Such patients report that they have pain but are not bothered by it; they recognize the sensation of pain but suffer little, or not at all.[26] Indifference to pain can also rarely be present from birth; these people have normal nerves on medical investigations, and find pain unpleasant, but do not avoid repetition of the pain stimulus.[27]
Insensitivity to pain may also result from abnormalities in the nervous system. This is usually the result of acquired damage to the nerves, such as spinal cord injury, diabetes mellitus (diabetic neuropathy), or leprosy in countries where that disease is prevalent.[28] These individuals are at risk of tissue damage and infection due to undiscovered injuries. People with diabetes-related nerve damage, for instance, sustain poorly-healing foot ulcers as a result of decreased sensation.[29]
A much smaller number of people are insensitive to pain due to an inborn abnormality of the nervous system, known as "congenital insensitivity to pain".[27] Children with this condition incur carelessly-repeated damage to their tongues, eyes, joints, skin, and muscles. Some die before adulthood, and others have a reduced life expectancy. Most people with congenital insensitivity to pain have one of five hereditary sensory and autonomic neuropathies (which includes familial dysautonomia and congenital insensitivity to pain with anhidrosis).[30] These conditions feature decreased sensitivity to pain together with other neurological abnormalities, particularly of the autonomic nervous system.[27][30] A very rare syndrome with isolated congenital insensitivity to pain has been linked with mutations in the SCN9A gene, which codes for a sodium channel (Nav1.7) necessary in conducting pain nerve stimuli.[31]
Functional effects
Experimental subjects challenged by acute pain and patients in chronic pain experience impairments in attention control, working memory, mental flexibility, problem solving, and information processing speed.[32] Acute and chronic pain are also associated with increased depression, anxiety, fear, and anger.[33]
If I have matters right, the consequences of pain will include direct physical distress, unemployment, financial difficulties, marital disharmony, and difficulties in concentration and attention…
— Harold Merskey 2000[34]
On subsequent negative emotion
Although pain is considered to be aversive and unpleasant and is therefore usually avoided, a meta-analysis which summarized and evaluated numerous studies from various psychological disciplines, found a reduction in negative affect. Across studies, participants that were subjected to acute physical pain in the laboratory subsequently reported feeling better than those in non-painful control conditions, a finding which was also reflected in physiological parameters.[35] A potential mechanism to explain this effect is provided by the opponent-process theory.
Theory
Historical
Before the relatively recent discovery of neurons and their role in pain, various different body functions were proposed to account for pain. There were several competing early theories of pain among the ancient Greeks: Hippocrates believed that it was due to an imbalance in vital fluids.[36] In the 11th century, Avicenna theorized that there were a number of feeling senses including touch, pain and titillation.[37]

In 1644, René Descartes theorized that pain was a disturbance that passed down along nerve fibers until the disturbance reached the brain.[36][38] Descartes's work, along with Avicenna's, prefigured the 19th-century development of specificity theory. Specificity theory saw pain as "a specific sensation, with its own sensory apparatus independent of touch and other senses".[39] Another theory that came to prominence in the 18th and 19th centuries was intensive theory, which conceived of pain not as a unique sensory modality, but an emotional state produced by stronger than normal stimuli such as intense light, pressure or temperature.[40] By the mid-1890s, specificity was backed mostly by physiologists and physicians, and the intensive theory was mostly backed by psychologists. However, after a series of clinical observations by Henry Head and experiments by Max von Frey, the psychologists migrated to specificity almost en masse, and by century's end, most textbooks on physiology and psychology were presenting pain specificity as fact.[37][39]
Modern
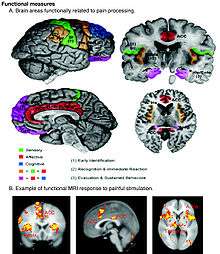
Wilhelm Erb's (1874) "intensive" theory, that a pain signal can be generated by intense enough stimulation of any sensory receptor, has been soundly disproved. Some sensory fibers do not differentiate between noxious and non-noxious stimuli, while others, nociceptors, respond only to noxious, high intensity stimuli. At the peripheral end of the nociceptor, noxious stimuli generate currents that, above a given threshold, send signals along the nerve fiber to the spinal cord. The "specificity" (whether it responds to thermal, chemical or mechanical features of its environment) of a nociceptor is determined by which ion channels it expresses at its peripheral end. Dozens of different types of nociceptor ion channels have so far been identified, and their exact functions are still being determined.[41]
The pain signal travels from the periphery to the spinal cord along an A-delta or C fiber. Because the A-delta fiber is thicker than the C fiber, and is thinly sheathed in an electrically insulating material (myelin), it carries its signal faster (5–30 m/s) than the unmyelinated C fiber (0.5–2 m/s).[42] Pain evoked by the A-delta fibers is described as sharp and is felt first. This is followed by a duller pain, often described as burning, carried by the C fibers.[43] These "first order" neurons enter the spinal cord via Lissauer's tract.
These A-delta and C fibers connect with "second order" nerve fibers in the central gelatinous substance of the spinal cord (laminae II and III of the dorsal horns). The second order fibers then cross the cord via the anterior white commissure and ascend in the spinothalamic tract. Before reaching the brain, the spinothalamic tract splits into the lateral, neospinothalamic tract and the medial, paleospinothalamic tract.[44]
Second order, spinal cord fibers dedicated to carrying A-delta fiber pain signals, and others that carry both A-delta and C fiber pain signals to the thalamus have been identified. Other spinal cord fibers, known as wide dynamic range neurons, respond to A-delta and C fibers, but also to the large A-beta fibers that carry touch, pressure and vibration signals.[42] Pain-related activity in the thalamus spreads to the insular cortex (thought to embody, among other things, the feeling that distinguishes pain from other homeostatic emotions such as itch and nausea) and anterior cingulate cortex (thought to embody, among other things, the affective/motivational element, the unpleasantness of pain).[45] Pain that is distinctly located also activates primary and secondary somatosensory cortex.[46]
In 1955, DC Sinclair and G Weddell developed peripheral pattern theory, based on a 1934 suggestion by John Paul Nafe. They proposed that all skin fiber endings (with the exception of those innervating hair cells) are identical, and that pain is produced by intense stimulation of these fibers.[39] Another 20th-century theory was gate control theory, introduced by Ronald Melzack and Patrick Wall in the 1965 Science article "Pain Mechanisms: A New Theory".[47] The authors proposed that both thin (pain) and large diameter (touch, pressure, vibration) nerve fibers carry information from the site of injury to two destinations in the dorsal horn of the spinal cord, and that the more large fiber activity relative to thin fiber activity at the inhibitory cell, the less pain is felt.[38]
Three dimensions of pain
In 1968 Ronald Melzack and Kenneth Casey described chronic pain in terms of its three dimensions:
- "sensory-discriminative" (sense of the intensity, location, quality and duration of the pain),
- "affective-motivational" (unpleasantness and urge to escape the unpleasantness), and
- "cognitive-evaluative" (cognitions such as appraisal, cultural values, distraction and hypnotic suggestion).
They theorized that pain intensity (the sensory discriminative dimension) and unpleasantness (the affective-motivational dimension) are not simply determined by the magnitude of the painful stimulus, but "higher" cognitive activities can influence perceived intensity and unpleasantness. Cognitive activities "may affect both sensory and affective experience or they may modify primarily the affective-motivational dimension. Thus, excitement in games or war appears to block both dimensions of pain, while suggestion and placebos may modulate the affective-motivational dimension and leave the sensory-discriminative dimension relatively undisturbed." (p. 432) The paper ends with a call to action: "Pain can be treated not only by trying to cut down the sensory input by anesthetic block, surgical intervention and the like, but also by influencing the motivational-affective and cognitive factors as well." (p. 435)
Evolutionary and behavioral role
Pain is part of the body's defense system, producing a reflexive retraction from the painful stimulus, and tendencies to protect the affected body part while it heals, and avoid that harmful situation in the future.[48][49] It is an important part of animal life, vital to healthy survival. People with congenital insensitivity to pain have reduced life expectancy.[27]
In The Greatest Show on Earth: The Evidence for Evolution, biologist Richard Dawkins addresses the question of why pain should have the quality of being painful. He describes the alternative as a mental raising of a "red flag". To argue why that red flag might be insufficient, Dawkins argues that drives must compete with one other within living beings. The most "fit" creature would be the one whose pains are well balanced. Those pains which mean certain death when ignored will become the most powerfully felt. The relative intensities of pain, then, may resemble the relative importance of that risk to our ancestors.[lower-alpha 1] This resemblance will not be perfect, however, because natural selection can be a poor designer. This may have maladaptive results such as supernormal stimuli.[50]
Pain, however, does not only wave a "red flag" within living beings but may also act as a warning sign and a call for help to other living beings. Especially in humans who readily helped each other in case of sickness or injury throughout their evolutionary history, pain might be shaped by natural selection to be a credible and convincing signal of need for relief, help, and care.[51]
Idiopathic pain (pain that persists after the trauma or pathology has healed, or that arises without any apparent cause) may be an exception to the idea that pain is helpful to survival, although some psychodynamic psychologists argue that such pain is psychogenic, enlisted as a protective distraction to keep dangerous emotions unconscious.[52]
Thresholds
In pain science, thresholds are measured by gradually increasing the intensity of a stimulus in a procedure called quantitative sensory testing which involves such stimuli as electric current, thermal (heat or cold), mechanical (pressure, touch, vibration), ischemic, or chemical stimuli applied to the subject to evoke a response.[53] The "pain perception threshold" is the point at which the subject begins to feel pain, and the "pain threshold intensity" is the stimulus intensity at which the stimulus begins to hurt. The "pain tolerance threshold" is reached when the subject acts to stop the pain.[53]
Assessment
A person's self-report is the most reliable measure of pain.[54][55][56] Some health care professionals may underestimate pain severity.[57] A definition of pain widely employed in nursing, emphasizing its subjective nature and the importance of believing patient reports, was introduced by Margo McCaffery in 1968: "Pain is whatever the experiencing person says it is, existing whenever he says it does".[58] To assess intensity, the patient may be asked to locate their pain on a scale of 0 to 10, with 0 being no pain at all, and 10 the worst pain they have ever felt. Quality can be established by having the patient complete the McGill Pain Questionnaire indicating which words best describe their pain.[6]
Visual analogue scale
The visual analogue scale is a common, reproducible tool in the assessment of pain and pain relief.[59] The scale is a continuous line anchored by verbal descriptors, one for each extreme of pain where a higher score indicates greater pain intensity. It is usually 10 cm in length with no intermediate descriptors as to avoid marking of scores around a preferred numeric value. When applied as a pain descriptor, these anchors are often 'no pain' and 'worst imaginable pain". Cut-offs for pain classification have been recommended as no pain (0-4mm), mild pain (5-44mm), moderate pain (45-74mm) and severe pain (75-100mm).[60]
Multidimensional pain inventory
The Multidimensional Pain Inventory (MPI) is a questionnaire designed to assess the psychosocial state of a person with chronic pain. Combining the MPI characterization of the person with their IASP five-category pain profile is recommended for deriving the most useful case description.[11]
Assessment in non-verbal people
Non-verbal people cannot use words to tell others that they are experiencing pain. However, they may be able to communicate through other means, such as blinking, pointing, or nodding.[61]
With a non-communicative person, observation becomes critical, and specific behaviors can be monitored as pain indicators. Behaviors such as facial grimacing and guarding (trying to protect part of the body from being bumped or touched) indicate pain, as well as an increase or decrease in vocalizations, changes in routine behavior patterns and mental status changes. Patients experiencing pain may exhibit withdrawn social behavior and possibly experience a decreased appetite and decreased nutritional intake. A change in condition that deviates from baseline, such as moaning with movement or when manipulating a body part, and limited range of motion are also potential pain indicators. In patients who possess language but are incapable of expressing themselves effectively, such as those with dementia, an increase in confusion or display of aggressive behaviors or agitation may signal that discomfort exists, and further assessment is necessary. Changes in behavior may be noticed by caregivers who are familiar with the person's normal behavior.[61]
Infants do feel pain, but lack the language needed to report it, and so communicate distress by crying. A non-verbal pain assessment should be conducted involving the parents, who will notice changes in the infant which may not be obvious to the health care provider. Pre-term babies are more sensitive to painful stimuli than those carried to full term.[62]
Another approach, when pain is suspected, is to give the person treatment for pain, and then watch to see whether the suspected indicators of pain subside.[61]
Other reporting barriers
The way in which one experiences and responds to pain is related to sociocultural characteristics, such as gender, ethnicity, and age.[63][64] An aging adult may not respond to pain in the same way that a younger person might. Their ability to recognize pain may be blunted by illness or the use of medication. Depression may also keep older adult from reporting they are in pain. Decline in self-care may also indicate the older adult is experiencing pain. They may be reluctant to report pain because they do not want to be perceived as weak, or may feel it is impolite or shameful to complain, or they may feel the pain is a form of deserved punishment.[65][66]
Cultural barriers may also affect the likelihood of reporting pain. Sufferers may feel that certain treatments go against their religious beliefs. They may not report pain because they feel it is a sign that death is near. Many people fear the stigma of addiction, and avoid pain treatment so as not to be prescribed potentially addicting drugs. Many Asians do not want to lose respect in society by admitting they are in pain and need help, believing the pain should be borne in silence, while other cultures feel they should report pain immediately to receive immediate relief.[62]
Gender can also be a perceived factor in reporting pain. Gender differences can be the result of social and cultural expectations, with women expected to be more emotional and show pain, and men more stoic.[62] As a result, female pain is often stigmatized, leading to less urgent treatment of women based on social expectations of their ability to accurately report it.[67] This leads to extended emergency room wait times for women and frequent dismissal of their ability to accurately report pain.[68][69]
Diagnostic aid
Pain is a symptom of many medical conditions. Knowing the time of onset, location, intensity, pattern of occurrence (continuous, intermittent, etc.), exacerbating and relieving factors, and quality (burning, sharp, etc.) of the pain will help the examining physician to accurately diagnose the problem. For example, chest pain described as extreme heaviness may indicate myocardial infarction, while chest pain described as tearing may indicate aortic dissection.[70][71]
Physiological measurement
Functional magnetic resonance imaging brain scanning has been used to measure pain, and correlates well with self-reported pain.[72][73][74]
Mechanism
Nociceptive
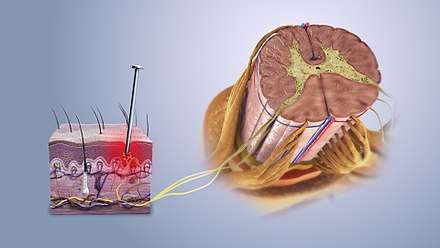
Nociceptive pain is caused by stimulation of sensory nerve fibers that respond to stimuli approaching or exceeding harmful intensity (nociceptors), and may be classified according to the mode of noxious stimulation. The most common categories are "thermal" (e.g. heat or cold), "mechanical" (e.g. crushing, tearing, shearing, etc.) and "chemical" (e.g. iodine in a cut or chemicals released during inflammation). Some nociceptors respond to more than one of these modalities and are consequently designated polymodal.
Nociceptive pain may also be classed according to the site of origin and divided into "visceral", "deep somatic" and "superficial somatic" pain. Visceral structures (e.g., the heart, liver and intestines) are highly sensitive to stretch, ischemia and inflammation, but relatively insensitive to other stimuli that normally evoke pain in other structures, such as burning and cutting. Visceral pain is diffuse, difficult to locate and often referred to a distant, usually superficial, structure. It may be accompanied by nausea and vomiting and may be described as sickening, deep, squeezing, and dull.[75] Deep somatic pain is initiated by stimulation of nociceptors in ligaments, tendons, bones, blood vessels, fasciae and muscles, and is dull, aching, poorly-localized pain. Examples include sprains and broken bones. Superficial somatic pain is initiated by activation of nociceptors in the skin or other superficial tissue, and is sharp, well-defined and clearly located. Examples of injuries that produce superficial somatic pain include minor wounds and minor (first degree) burns.[12]
Neuropathic
Neuropathic pain is caused by damage or disease affecting any part of the nervous system involved in bodily feelings (the somatosensory system).[76] Neuropathic pain may be divided into peripheral, central, or mixed (peripheral and central) neuropathic pain. Peripheral neuropathic pain is often described as "burning", "tingling", "electrical", "stabbing", or "pins and needles".[77] Bumping the "funny bone" elicits acute peripheral neuropathic pain.
Nociplastic
Nociplastic pain is pain characterized by a changed nociception (but without evidence of real or threatened tissue damage, or without disease or damage in the somatosensory system).[78]
This applies, for example, to fibromyalgia patients.
Psychogenic
Psychogenic pain, also called psychalgia or somatoform pain, is pain caused, increased, or prolonged by mental, emotional, or behavioral factors.[79] Headache, back pain, and stomach pain are sometimes diagnosed as psychogenic.[79] Sufferers are often stigmatized, because both medical professionals and the general public tend to think that pain from a psychological source is not "real". However, specialists consider that it is no less actual or hurtful than pain from any other source.[25]
People with long-term pain frequently display psychological disturbance, with elevated scores on the Minnesota Multiphasic Personality Inventory scales of hysteria, depression and hypochondriasis (the "neurotic triad"). Some investigators have argued that it is this neuroticism that causes acute pain to turn chronic, but clinical evidence points the other direction, to chronic pain causing neuroticism. When long-term pain is relieved by therapeutic intervention, scores on the neurotic triad and anxiety fall, often to normal levels. Self-esteem, often low in chronic pain patients, also shows improvement once pain has resolved.[19]:31–2
Management
Inadequate treatment of pain is widespread throughout surgical wards, intensive care units, and accident and emergency departments, in general practice, in the management of all forms of chronic pain including cancer pain, and in end of life care.[80][81][82][83][84][85][86] This neglect extends to all ages, from newborns to medically frail elderly.[87][88] African and Hispanic Americans are more likely than others to suffer unnecessarily while in the care of a physician;[89][90] and women's pain is more likely to be undertreated than men's.[91]
The International Association for the Study of Pain advocates that the relief of pain should be recognized as a human right, that chronic pain should be considered a disease in its own right, and that pain medicine should have the full status of a medical specialty.[92] It is a specialty only in China and Australia at this time.[93] Elsewhere, pain medicine is a subspecialty under disciplines such as anesthesiology, physiatry, neurology, palliative medicine and psychiatry.[94] In 2011, Human Rights Watch alerted that tens of millions of people worldwide are still denied access to inexpensive medications for severe pain.[95]
Medication
Acute pain is usually managed with medications such as analgesics and anesthetics.[96] Caffeine when added to pain medications such as ibuprofen, may provide some additional benefit.[97][98] Ketamine can be used instead of opioids for short term pain.[99] Management of chronic pain, however, is more difficult, and may require the coordinated efforts of a pain management team, which typically includes medical practitioners, clinical pharmacists, clinical psychologists, physiotherapists, occupational therapists, physician assistants, and nurse practitioners.[100]
Sugar (sucrose) when taken by mouth reduces pain in newborn babies undergoing some medical procedures (a lancing of the heel, venipuncture, and intramuscular injections). Sugar does not remove pain from circumcision, and it is unknown if sugar reduces pain for other procedures.[101] Sugar did not affect pain-related electrical activity in the brains of newborns one second after the heel lance procedure.[102] Sweet liquid by mouth moderately reduces the rate and duration of crying caused by immunization injection in children between one and twelve months of age.[103]
Psychological
Individuals with more social support experience less cancer pain, take less pain medication, report less labor pain and are less likely to use epidural anesthesia during childbirth, or suffer from chest pain after coronary artery bypass surgery.[8]
Suggestion can significantly affect pain intensity. About 35% of people report marked relief after receiving a saline injection they believed to be morphine. This placebo effect is more pronounced in people who are prone to anxiety, and so anxiety reduction may account for some of the effect, but it does not account for all of it. Placebos are more effective for intense pain than mild pain; and they produce progressively weaker effects with repeated administration.[19]:26–8 It is possible for many with chronic pain to become so absorbed in an activity or entertainment that the pain is no longer felt, or is greatly diminished.[19]:22–3
Cognitive behavioral therapy (CBT) has been shown effective for improving quality of life in those with chronic pain but the reduction in suffering is modest, and the CBT method was not shown to have any effect on outcome.[104] Acceptance and commitment therapy (ACT) may also be effective in the treatment of chronic pain,[105] as may mindfulness-based pain management (MBPM).[106][107][108]
A number of meta-analyses have found clinical hypnosis to be effective in controlling pain associated with diagnostic and surgical procedures in both adults and children, as well as pain associated with cancer and childbirth.[109] A 2007 review of 13 studies found evidence for the efficacy of hypnosis in the reduction of chronic pain under some conditions, though the number of patients enrolled in the studies was low, raising issues related to the statistical power to detect group differences, and most lacked credible controls for placebo or expectation. The authors concluded that "although the findings provide support for the general applicability of hypnosis in the treatment of chronic pain, considerably more research will be needed to fully determine the effects of hypnosis for different chronic-pain conditions."[110]
Alternative medicine
An analysis of the 13 highest quality studies of pain treatment with acupuncture, published in January 2009, concluded there was little difference in the effect of real, faked and no acupuncture.[111] However, more recent reviews have found some benefit.[112][113][114] Additionally, there is tentative evidence for a few herbal medicines.[115] There has been some interest in the relationship between vitamin D and pain, but the evidence so far from controlled trials for such a relationship, other than in osteomalacia, is inconclusive.[116]
For chronic (long-term) lower back pain, spinal manipulation produces tiny, clinically insignificant, short-term improvements in pain and function, compared sham therapy and other interventions.[117] Spinal manipulation produces the same outcome as other treatments, such as general practitioner care, pain-relief drugs, physical therapy, and exercise, for acute (short-term) lower back pain.[117]
Epidemiology
Pain is the main reason for visiting an emergency department in more than 50% of cases,[118] and is present in 30% of family practice visits.[119] Several epidemiological studies have reported widely varying prevalence rates for chronic pain, ranging from 12 to 80% of the population.[120] It becomes more common as people approach death. A study of 4,703 patients found that 26% had pain in the last two years of life, increasing to 46% in the last month.[121]
A survey of 6,636 children (0–18 years of age) found that, of the 5,424 respondents, 54% had experienced pain in the preceding three months. A quarter reported having experienced recurrent or continuous pain for three months or more, and a third of these reported frequent and intense pain. The intensity of chronic pain was higher for girls, and girls' reports of chronic pain increased markedly between ages 12 and 14.[122]
History
In 1994, responding to the need for a more useful system for describing chronic pain, the International Association for the Study of Pain (IASP) classified pain according to specific characteristics:
- region of the body involved (e.g. abdomen, lower limbs),
- system whose dysfunction may be causing the pain (e.g., nervous, gastrointestinal),
- duration and pattern of occurrence,
- intensity and time since onset, and
- cause[123]
However, this system has been criticized by Clifford J. Woolf and others as inadequate for guiding research and treatment.[124] Woolf suggests three classes of pain:
- nociceptive pain,
- inflammatory pain which is associated with tissue damage and the infiltration of immune cells, and
- pathological pain which is a disease state caused by damage to the nervous system or by its abnormal function (e.g. fibromyalgia, peripheral neuropathy, tension type headache, etc.).[125]
Society and culture

The nature or meaning of physical pain has been diversely understood by religious or secular traditions from antiquity to modern times.[126][127]
Physical pain is an important political topic in relation to various issues, including pain management policy, drug control, animal rights or animal welfare, torture, and pain compliance. In various contexts, the deliberate infliction of pain in the form of corporal punishment is used as retribution for an offence, or for the purpose of disciplining or reforming a wrongdoer, or to deter attitudes or behaviour deemed unacceptable. The slow slicing, or death by a thousand cuts, was a form of execution in China reserved for crimes viewed as especially severe, such as high treason or patricide. In some cultures, extreme practices such as mortification of the flesh or painful rites of passage are highly regarded. For example, the Sateré-Mawé people of Brazil use intentional bullet ant stings as part of their initiation rites to become warriors.[128]
Non-humans
The most reliable method for assessing pain in most humans is by asking a question: a person may report pain that cannot be detected by any known physiological measure. However, like infants, animals cannot answer questions about whether they feel pain; thus the defining criterion for pain in humans cannot be applied to them. Philosophers and scientists have responded to this difficulty in a variety of ways. René Descartes for example argued that animals lack consciousness and therefore do not experience pain and suffering in the way that humans do.[129] Bernard Rollin of Colorado State University, the principal author of two U.S. federal laws regulating pain relief for animals,[lower-alpha 2] writes that researchers remained unsure into the 1980s as to whether animals experience pain, and that veterinarians trained in the U.S. before 1989 were simply taught to ignore animal pain.[131] In his interactions with scientists and other veterinarians, he was regularly asked to "prove" that animals are conscious, and to provide "scientifically acceptable" grounds for claiming that they feel pain.[131] Carbone writes that the view that animals feel pain differently is now a minority view. Academic reviews of the topic are more equivocal, noting that although the argument that animals have at least simple conscious thoughts and feelings has strong support,[132] some critics continue to question how reliably animal mental states can be determined.[129][133] The ability of invertebrate species of animals, such as insects, to feel pain and suffering is also unclear.[134][135][136]
The presence of pain in an animal cannot be known for certain, but it can be inferred through physical and behavioral reactions.[137] Specialists currently believe that all vertebrates can feel pain, and that certain invertebrates, like the octopus, may also.[134][138][139] As for other animals, plants, or other entities, their ability to feel physical pain is at present a question beyond scientific reach, since no mechanism is known by which they could have such a feeling. In particular, there are no known nociceptors in groups such as plants, fungi, and most insects,[140] except for instance in fruit flies.[141]
In vertebrates, endogenous opioids are neuromodulators that moderate pain by interacting with opioid receptors.[142] Opioids and opioid receptors occur naturally in crustaceans and, although at present no certain conclusion can be drawn,[143] their presence indicates that lobsters may be able to experience pain.[143][144] Opioids may mediate their pain in the same way as in vertebrates.[144] Veterinary medicine uses, for actual or potential animal pain, the same analgesics and anesthetics as used in humans.[145]
Etymology
First attested in English in 1297, the word peyn comes from the Old French peine, in turn from Latin poena meaning "punishment, penalty"[146] (in L.L. also meaning "torment, hardship, suffering") and that from Greek ποινή (poine), generally meaning "price paid, penalty, punishment".[147][148]
See also
- Hedonic adaptation, the tendency to quickly return to a relatively stable level of happiness despite major positive or negative events
- Pain and suffering, the legal term for the physical and emotional stress caused from an injury
- Pain (philosophy), the branch of philosophy concerned with suffering and physical pain
Notes
- For example, lack of food, extreme cold, or serious injuries are felt as exceptionally painful, whereas minor damage is felt as mere discomfort
- Rollin drafted the 1985 Health Research Extension Act and an animal welfare amendment to the 1985 Food Security Act.[130]
References
- Raja, Srinivasa N.; Carr, Daniel B.; Cohen, Milton; Finnerup, Nanna B.; Flor, Herta; Gibson, Stephen; Keefe, Francis J.; Mogil, Jeffrey S.; Ringkamp, Matthias; Sluka, Kathleen A.; Song, Xue-Jun (21 July 2020). "The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises". PAIN. Articles in Press. doi:10.1097/j.pain.0000000000001939. ISSN 0304-3959.
- Cervero, Fernando (2012). Understanding Pain : Exploring the Perception of Pain. Cambridge, Mass.: MIT Press. pp. Chapter 1. ISBN 9780262305433. OCLC 809043366.
- Raj PP (2007). "Taxonomy and classification of pain". In: The Handbook of Chronic Pain. Nova Biomedical Books. ISBN 9781600210440.
- Debono DJ, Hoeksema LJ, Hobbs RD (August 2013). "Caring for patients with chronic pain: pearls and pitfalls". The Journal of the American Osteopathic Association. 113 (8): 620–7. doi:10.7556/jaoa.2013.023. PMID 23918913.
- Turk DC, Dworkin RH (2004). "What should be the core outcomes in chronic pain clinical trials?". Arthritis Research & Therapy. 6 (4): 151–4. doi:10.1186/ar1196. PMC 464897. PMID 15225358.
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A (July 2008). "Assessment of pain". British Journal of Anaesthesia. 101 (1): 17–24. doi:10.1093/bja/aen103. PMID 18487245.
- Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L (November 2015). "Non-prescription (OTC) oral analgesics for acute pain - an overview of Cochrane reviews". The Cochrane Database of Systematic Reviews. 11 (11): CD010794. doi:10.1002/14651858.CD010794.pub2. PMC 6485506. PMID 26544675.
- Eisenberger NI, Lieberman M (2005). "Why it hurts to be left out: The neurocognitive overlap between physical and social pain" (PDF). In Williams KD (ed.). The Social Outcast: Ostracism, Social Exclusion, Rejection, & Bullying (Sydney Symposium of Social Psychology). East Sussex: Psychology Press. p. 210. ISBN 9781841694245.
- Garland, Eric L.; Brintz, Carrie E.; Hanley, Adam W.; Roseen, Eric J.; Atchley, Rachel M.; Gaylord, Susan A.; Faurot, Keturah R.; Yaffe, Joanne; Fiander, Michelle; Keefe, Francis J. (1 January 2020). "Mind-Body Therapies for Opioid-Treated Pain". JAMA Internal Medicine. 180 (1): 91. doi:10.1001/jamainternmed.2019.4917. PMID 31682676.
- Weyers H (September 2006). "Explaining the emergence of euthanasia law in the Netherlands: how the sociology of law can help the sociology of bioethics". Sociology of Health & Illness. 28 (6): 802–16. doi:10.1111/j.1467-9566.2006.00543.x. PMID 17184419.
- Turk DC, Okifuji A (2001). "Pain terms and taxonomies of pain". In Bonica JJ, Loeser JD, Chapman CR, Turk DC (eds.). Bonica's management of pain. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 9780781768276.
- Coda BA, Bonica JJ (2000). "General considerations of acute pain". In Panswick CC, Main CJ (eds.). Pain management: an interdisciplinary approach. Edinburgh: Churchill Livingstone. ISBN 9780443056833.
- Thienhaus O, Cole BE (2002). "Classification of pain". In Weiner R (ed.). Pain management: a practical guide for clinicians. Boca Raton: CRC Press. pp. 28. ISBN 9780849322624.
- Jensen TS, Finnerup NB (September 2014). "Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms". The Lancet. Neurology. 13 (9): 924–35. doi:10.1016/s1474-4422(14)70102-4. PMID 25142459.
- Lolignier S, Eijkelkamp N, Wood JN (January 2015). "Mechanical allodynia". Pflügers Archiv. 467 (1): 133–9. doi:10.1007/s00424-014-1532-0. PMC 4281368. PMID 24846747.
- Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP (July 2000). "Phantom pain and phantom sensations in upper limb amputees: an epidemiological study". Pain. 87 (1): 33–41. doi:10.1016/S0304-3959(00)00264-5. PMID 10863043.
- Jensen TS, Krebs B, Nielsen J, Rasmussen P (November 1983). "Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation". Pain. 17 (3): 243–56. doi:10.1016/0304-3959(83)90097-0. PMID 6657285.
- Jensen TS, Krebs B, Nielsen J, Rasmussen P (March 1985). "Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain". Pain. 21 (3): 267–78. doi:10.1016/0304-3959(85)90090-9. PMID 3991231.
- Wall PD, Melzack R (1996). The challenge of pain (2nd ed.). New York: Penguin Books. ISBN 9780140256703.
- Ramachandran VS, Rogers-Ramachandran D (April 1996). "Synaesthesia in phantom limbs induced with mirrors". Proceedings. Biological Sciences. 263 (1369): 377–86. Bibcode:1996RSPSB.263..377R. doi:10.1098/rspb.1996.0058. PMID 8637922.
- Mishra S, Bhatnagar S, Chaudhary P, Rana SP (January 2009). "Breakthrough cancer pain: review of prevalence, characteristics and management". Indian Journal of Palliative Care. 15 (1): 14–8. doi:10.4103/0973-1075.53506. PMC 2886208. PMID 20606850.
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G (February 2012). "Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC" (PDF). The Lancet. Oncology. 13 (2): e58-68. doi:10.1016/S1470-2045(12)70040-2. PMID 22300860. Archived from the original (PDF) on 19 October 2014.
- Beecher HK (1959). Measurement of subjective responses. New York: Oxford University Press. cited in Melzack R, Wall PD (1996). The challenge of pain (2nd ed.). London: Penguin. p. 7. ISBN 978-0-14-025670-3.
- "International Association for the Study of Pain: Pain Definitions". Archived from the original on 13 January 2015. Retrieved 12 January 2015.
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage
Alt URL Derived from Bonica JJ (June 1979). "The need of a taxonomy". Pain. 6 (3): 247–8. doi:10.1016/0304-3959(79)90046-0. PMID 460931. - "International Association for the Study of Pain | Pain Definitions".. Retrieved 12 October 2010.
- Nikola Grahek, Feeling pain and being in pain Archived 27 September 2008 at the Wayback Machine, Oldenburg, 2001. ISBN 9780262517324.
- Nagasako EM, Oaklander AL, Dworkin RH (February 2003). "Congenital insensitivity to pain: an update". Pain. 101 (3): 213–9. doi:10.1016/S0304-3959(02)00482-7. PMID 12583863.
- Brand PW, Yancey P (1997). The gift of pain: why we hurt & what we can do about it. Grand Rapids, Mich: Zondervan Publ. ISBN 9780310221449.
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS (October 2004). "Diagnosis and treatment of diabetic foot infections". Clinical Infectious Diseases. 39 (7): 885–910. doi:10.1086/424846. PMID 15472838.
- Axelrod FB, Hilz MJ (December 2003). "Inherited autonomic neuropathies". Seminars in Neurology. 23 (4): 381–90. doi:10.1055/s-2004-817722. PMID 15088259.
- Raouf R, Quick K, Wood JN (November 2010). "Pain as a channelopathy". The Journal of Clinical Investigation. 120 (11): 3745–52. doi:10.1172/JCI43158. PMC 2965577. PMID 21041956.
- Hart RP, Wade JB, Martelli MF (April 2003). "Cognitive impairment in patients with chronic pain: the significance of stress". Current Pain and Headache Reports. 7 (2): 116–26. doi:10.1007/s11916-003-0021-5. PMID 12628053.
- Bruehl S, Burns JW, Chung OY, Chont M (March 2009). "Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms". Neuroscience and Biobehavioral Reviews. 33 (3): 475–91. doi:10.1016/j.neubiorev.2008.12.003. PMC 2756489. PMID 19146872.
- Merskey H (2000). "The History of Psychoanalitic Ideas Concerning Pain". In Weisberg JN, Gatchel RJ (eds.). Personality Characteristics of Patients With Pain. American Psychological Association (APA). ISBN 978-1-55798-646-7.
- Bresin K, Kling L, Verona E (2018). "The effect of acute physical pain on subsequent negative emotional affect: A meta-analysis". Personality Disorders: Theory, Research, and Treatment. 9 (3): 273–283. doi:10.1037/per0000248. PMC 5624817. PMID 28368146.CS1 maint: multiple names: authors list (link)
- Linton. Models of Pain Perception. Elsevier Health, 2005. Print.
- Dallenbach KM (July 1939). "Pain: History and present status". American Journal of Psychology. 52 (3): 331–347. doi:10.2307/1416740. JSTOR 1416740.
- Melzack R, Katz J (2004). "The Gate Control Theory: Reaching for the Brain". In Craig KD, Hadjistavropoulos T (eds.). Pain: psychological perspectives. Mahwah, N.J: Lawrence Erlbaum Associates, Publishers. ISBN 9780415650618.
- Bonica JJ (1990). "History of pain concepts and therapies". The management of pain. 1 (2 ed.). London: Lea & Febiger. p. 7. ISBN 9780812111224.
- Finger S (2001). Origins of neuroscience: a history of explorations into brain function. USA: Oxford University Press. p. 149. ISBN 9780195146943.
- Woolf CJ, Ma Q (August 2007). "Nociceptors--noxious stimulus detectors". Neuron. 55 (3): 353–64. doi:10.1016/j.neuron.2007.07.016. PMID 17678850.
- Marchand S (2010). "Applied pain neurophysiology". In Beaulieu P, Lussier D, Porreca F, Dickenson A (eds.). Pharmacology of pain. Seattle: International Association for the Study of Pain Press. pp. 3–26. ISBN 978-0-931092-78-7.
- Skevington S (1995). Psychology of pain. New York: Wiley. p. 9. ISBN 9780471957737.
- Skevington SM (1995). Psychology of pain. Chichester, UK: Wiley. p. 18. ISBN 9780471957737.
- Craig AD (2003). "Pain mechanisms: labeled lines versus convergence in central processing". Annual Review of Neuroscience. 26: 1–30. doi:10.1146/annurev.neuro.26.041002.131022. PMID 12651967.
- Romanelli P, Esposito V (July 2004). "The functional anatomy of neuropathic pain". Neurosurgery Clinics of North America. 15 (3): 257–68. doi:10.1016/j.nec.2004.02.010. PMID 15246335.
- Melzack R, Wall PD (November 1965). "Pain mechanisms: a new theory" (PDF). Science. 150 (3699): 971–9. Bibcode:1965Sci...150..971M. doi:10.1126/science.150.3699.971. PMID 5320816. Archived from the original (PDF) on 14 January 2012.
- Lynn B (1984). "Cutaneous nociceptors". In Winlow W, Holden AV (eds.). The neurobiology of pain: Symposium of the Northern Neurobiology Group, held at Leeds on 18 April 1983. Manchester: Manchester University Press. p. 106. ISBN 9780719009969.
- Bernston GG, Cacioppo JT (2007). "The neuroevolution of motivation". In Gardner WL, Shah JY (eds.). Handbook of Motivation Science. New York: The Guilford Press. p. 191. ISBN 9781593855680.
- Dawkins R (2009). The Greatest Show on Earth. Free Press. pp. 392–395.
- Steinkopf L (June 2016). "An Evolutionary Perspective on Pain Communication". Evolutionary Psychology. 14 (2): 100. doi:10.1177/1474704916653964.
- Sarno JE (2006). The divided mind: the epidemic of mindbody disorders. New York: ReganBooks. ISBN 9780061174308.
- Fillingim RB, Loeser JD, Baron R, Edwards RR (September 2016). "Assessment of Chronic Pain: Domains, Methods, and Mechanisms". The Journal of Pain. 17 (9 Suppl): T10-20. doi:10.1016/j.jpain.2015.08.010. PMC 5010652. PMID 27586827.
- Amico D (2016). Health & physical assessment in nursing. Boston: Pearson. p. 173. ISBN 9780133876406.
- Taylor C (2015). Fundamentals of nursing : the art and science of person-centered nursing care. Philadelphia: Wolters Kluwer Health. p. 241. ISBN 9781451185614.
- Venes D (2013). Taber's cyclopedic medical dictionary. Philadelphia: F.A. Davis. p. 1716. ISBN 9780803629776.
- Prkachin KM, Solomon PE, Ross J (June 2007). "Underestimation of pain by health-care providers: towards a model of the process of inferring pain in others". The Canadian Journal of Nursing Research. 39 (2): 88–106. PMID 17679587.
- McCaffery M. (1968). Nursing practice theories related to cognition, bodily pain, and man-environment interactions. Los Angeles: UCLA Students Store.
More recently, McCaffery defined pain as "whatever the experiencing person says it is, existing whenever the experiencing person says it does." Pasero C, McCaffery M (1999). Pain: clinical manual. St. Louis: Mosby. ISBN 9780815156093. - Kelly AM (May 2001). "The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain". Emergency Medicine Journal. 18 (3): 205–7. doi:10.1136/emj.18.3.205. PMC 1725574. PMID 11354213. Archived from the original on 25 January 2018.
- Hawker GA, Mian S, Kendzerska T, French M (November 2011). "Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP)". Arthritis Care & Research. 63 Suppl 11 (S11): S240-52. doi:10.1002/acr.20543. PMID 22588748.
- Lewis, Sharon Mantik; Bucher, Linda; Heitkemper, Margaret M. (Margaret McLean); Harding, Mariann (2017). Medical-surgical nursing: Assessment and management of clinical problems (10th ed.). St. Louis, Missouri: Elsevier. p. 126. ISBN 9780323328524. OCLC 944472408.
- Jarvis C (2007). Physical examination & health assessment. St. Louis, Mo: Elsevier Saunders. pp. 180–192. ISBN 9781455728107.
- Encandela JA (March 1993). "Social science and the study of pain since Zborowski: a need for a new agenda". Social Science & Medicine. 36 (6): 783–91. doi:10.1016/0277-9536(93)90039-7. PMID 8480223.
- Zborowski M. People in Pain. 1969, San Francisco, CA:Josey-Bass
- Encandela JA (1997). "Social Construction of pain and aging: Individual artfulness within interpretive structures". Symbolic Interaction. 20 (3): 251–273. doi:10.1525/si.1997.20.3.251.
- Lawhorne L, Passerini J (1999). Chronic Pain Management in the Long Term Care Setting: Clinical Practice Guidelines. Baltimore, Maryland: American Medical Directors Association. pp. 1–27.
- Epstein, Randy (19 March 2018). "When Doctors Don't Listen to Women". The New York Times. Retrieved 20 July 2019.
- Fasslet, Joe (15 October 2015). "How Doctors Take Women's Pain Less Seriously". The Atlantic. Retrieved 20 July 2019.
- "Stories of Misunderstanding Women's Pain". The Atlantic. 15 March 2016. Retrieved 20 July 2019.
- Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL (October 1998). "The rational clinical examination. Is this patient having a myocardial infarction?". JAMA. 280 (14): 1256–63. doi:10.1001/jama.280.14.1256. PMID 9786377.
- Slater EE, DeSanctis RW (May 1976). "The clinical recognition of dissecting aortic aneurysm". The American Journal of Medicine. 60 (5): 625–33. doi:10.1016/0002-9343(76)90496-4. PMID 1020750.
- Brown JE, Chatterjee N, Younger J, Mackey S (September 2011). "Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation". PLOS One. 6 (9): e24124. Bibcode:2011PLoSO...624124B. doi:10.1371/journal.pone.0024124. PMC 3172232. PMID 21931652.
- Paddock C (15 September 2011). "Tool That Measures Pain Objectively Under Way". Medical News Today. Archived from the original on 25 September 2017. Retrieved 25 September 2017.
- Reuters Editorial (13 September 2011). "Feeling pain? The computer can tell". Reuters. Archived from the original on 17 June 2015. Retrieved 25 September 2017.
- Urch CE, Suzuki R (26 September 2008). "Pathophysiology of somatic, visceral, and neuropathic cancer pain". In Sykes N, Bennett MI & Yuan C-S (ed.). Clinical pain management: Cancer pain (2 ed.). London: Hodder Arnold. pp. 3–12. ISBN 978-0-340-94007-5.
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J (April 2008). "Neuropathic pain: redefinition and a grading system for clinical and research purposes". Neurology. 70 (18): 1630–5. doi:10.1212/01.wnl.0000282763.29778.59. PMID 18003941.
- Paice JA (2003). "Mechanisms and management of neuropathic pain in cancer" (PDF). The Journal of Supportive Oncology. 1 (2): 107–20. PMID 15352654. Archived from the original (PDF) on 7 January 2010. Retrieved 8 January 2010.
- Kosek, Eva; Cohen, Milton; Baron, Ralf; Gebhart, Gerald F.; Mico, Juan-Antonio; Rice, Andrew S. C.; Rief, Winfried; Sluka, A. Kathleen (July 2016). "Do we need a third mechanistic descriptor for chronic pain states?". Pain. 157 (7): 1382–1386. doi:10.1097/j.pain.0000000000000507. PMID 26835783. Derived from Kosek E, et al. (July 2016). "Do we need a third mechanistic descriptor for chronic pain states?". Pain. 157 (7): 1382–6. doi:10.1097/j.pain.0000000000000507. PMID 26835783.
- "Psychogenic Pain". Cleveland Clinic. Archived from the original on 14 July 2011. Retrieved 25 September 2017.
- Brown AK, Christo PJ, Wu CL (December 2004). "Strategies for postoperative pain management". Best Practice & Research. Clinical Anaesthesiology. 18 (4): 703–17. doi:10.1016/j.bpa.2004.05.004. PMID 15460554.
- Cullen L, Greiner J, Titler MG (June 2001). "Pain management in the culture of critical care". Critical Care Nursing Clinics of North America. 13 (2): 151–66. doi:10.1016/S0899-5885(18)30046-7. PMID 11866399.
- Rupp T, Delaney KA (April 2004). "Inadequate analgesia in emergency medicine". Annals of Emergency Medicine. 43 (4): 494–503. doi:10.1016/j.annemergmed.2003.11.019. PMID 15039693.
- Smith GF, Toonen TR (April 2007). "Primary care of the patient with cancer". American Family Physician. 75 (8): 1207–14. PMID 17477104.
- Jacobson PL, Mann JD (January 2003). "Evolving role of the neurologist in the diagnosis and treatment of chronic noncancer pain". Mayo Clinic Proceedings. 78 (1): 80–4. doi:10.4065/78.1.80. PMID 12528880.
- Deandrea S, Montanari M, Moja L, Apolone G (December 2008). "Prevalence of undertreatment in cancer pain. A review of published literature". Annals of Oncology. 19 (12): 1985–91. doi:10.1093/annonc/mdn419. PMC 2733110. PMID 18632721.
- Perron V, Schonwetter RS (2001). "Assessment and management of pain in palliative care patients" (PDF). Cancer Control. 8 (1): 15–24. doi:10.1177/107327480100800103. PMID 11176032. Archived from the original (PDF) on 12 July 2008.
- Selbst SM, Fein JA (2006). "Sedation and analgesia". In Henretig FM, Fleisher GR, Ludwig S (eds.). Textbook of pediatric emergency medicine. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 9781605471594.
- Cleeland CS (June 1998). "Undertreatment of cancer pain in elderly patients". JAMA. 279 (23): 1914–5. doi:10.1001/jama.279.23.1914. PMID 9634265.
- Bonham VL (2001). "Race, ethnicity, and pain treatment: striving to understand the causes and solutions to the disparities in pain treatment" (PDF). The Journal of Law, Medicine & Ethics. 29 (1): 52–68. doi:10.1111/j.1748-720X.2001.tb00039.x. PMID 11521272. Archived (PDF) from the original on 19 July 2011.
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Kaloukalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH (September 2003). "The unequal burden of pain: confronting racial and ethnic disparities in pain" (PDF). Pain Medicine. 4 (3): 277–94. doi:10.1046/j.1526-4637.2003.03034.x. PMID 12974827.
- Hoffmann DE, Tarzian AJ (2001). "The girl who cried pain: a bias against women in the treatment of pain". The Journal of Law, Medicine & Ethics. 29 (1): 13–27. doi:10.1111/j.1748-720X.2001.tb00037.x. PMID 11521267.
- Delegates to the International Pain Summit of the International Association for the Study of Pain (2010) "Declaration of Montreal" Archived 13 May 2011 at the Wayback Machine.
- Horlocker TT, Cousins MJ, Bridenbaugh PO, Carr DL (2008). Cousins and Bridenbaugh's Neural Blockade in Clinical Anesthesia and Pain Medicine. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 9780781773881.
- "Physical Medicine and Rehabilitation" Archived 16 May 2008 at the Wayback Machine
- Human Rights Watch (2011). "Tens of Millions Face Death in Agony". Archived from the original on 1 September 2013. Retrieved 26 August 2013.
- Mallinson, Tom (2017). "A review of ketorolac as a prehospital analgesic". Journal of Paramedic Practice. 9 (12): 522–526. doi:10.12968/jpar.2017.9.12.522. Retrieved 2 June 2018.
- Derry CJ, Derry S, Moore RA (December 2014). "Caffeine as an analgesic adjuvant for acute pain in adults". The Cochrane Database of Systematic Reviews. 12 (12): CD009281. doi:10.1002/14651858.CD009281.pub3. PMC 6485702. PMID 25502052.
- Derry S, Wiffen PJ, Moore RA (July 2015). "Single dose oral ibuprofen plus caffeine for acute postoperative pain in adults". The Cochrane Database of Systematic Reviews (7): CD011509. doi:10.1002/14651858.CD011509.pub2. PMC 6481458. PMID 26171993.
- Karlow N, Schlaepfer CH, Stoll CR, Doering M, Carpenter CR, Colditz GA, Motov S, Miller J, Schwarz ES (October 2018). "A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department". Academic Emergency Medicine. 25 (10): 1086–1097. doi:10.1111/acem.13502. PMID 30019434.
- Thienhaus O, Cole BE (2002). "The classification of pain". In Weiner RS (ed.). Pain management: A practical guide for clinicians. American Academy of Pain Management. p. 29. ISBN 9780849322624.
- Main CJ, Spanswick CC (2000). Pain management: an interdisciplinary approach. Churchill Livingstone. ISBN 9780443056833.
Pain management: an interdisciplinary approach.
- Main CJ, Spanswick CC (2000). Pain management: an interdisciplinary approach. Churchill Livingstone. ISBN 9780443056833.
- Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A (July 2016). "Sucrose for analgesia in newborn infants undergoing painful procedures". The Cochrane Database of Systematic Reviews. 7: CD001069. doi:10.1002/14651858.CD001069.pub5. PMC 6457867. PMID 27420164.
- Lasky RE, van Drongelen W (October 2010). "Is sucrose an effective analgesic for newborn babies?". Lancet. 376 (9748): 1201–3. doi:10.1016/S0140-6736(10)61358-X. PMID 20817245.
- Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, Ohlsson A (June 2010). "Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review". Archives of Disease in Childhood. 95 (6): 406–13. doi:10.1136/adc.2009.174227. PMID 20463370.
- Vlaeyen JW, Morley S (2005). "Cognitive-behavioral treatments for chronic pain: what works for whom?". The Clinical Journal of Pain. 21 (1): 1–8. doi:10.1097/00002508-200501000-00001. PMID 15599126.
- Ost LG (October 2014). "The efficacy of Acceptance and Commitment Therapy: an updated systematic review and meta-analysis". Behaviour Research and Therapy. 61: 105–21. doi:10.1016/j.brat.2014.07.018. PMID 25193001.
- Mehan, Suraj; Morris, Julia (2018). "A literature review of Breathworks and mindfulness intervention". British Journal of Healthcare Management. 24 (5): 235–241. doi:10.12968/bjhc.2018.24.5.235. ISSN 1358-0574.
- J, Long; M, Briggs; A, Long; F, Astin (2016). "Starting Where I Am: A Grounded Theory Exploration of Mindfulness as a Facilitator of Transition in Living With a Long-Term Condition". Journal of advanced nursing. PMID 27174075. Retrieved 29 May 2020.
- Brown, CA; Jones, AKP (2013). "Psychobiological Correlates of Improved Mental Health in Patients With Musculoskeletal Pain After a Mindfulness-Based Pain Management Program". The Clinical journal of pain. PMID 22874090. Retrieved 29 May 2020.
- Wark, D.M. (2008). What can we do with hypnosis: A brief note. American Journal of Clinical Hypnosis. http://www.tandfonline.com/doi/abs/10.1080/00029157.2008.10401640#.UgGMqZLVArU
- Elkins G, Jensen MP, Patterson DR (July 2007). "Hypnotherapy for the management of chronic pain". The International Journal of Clinical and Experimental Hypnosis. 55 (3): 275–87. doi:10.1080/00207140701338621. PMC 2752362. PMID 17558718.
- Madsen MV, Gøtzsche PC, Hróbjartsson A (January 2009). "Acupuncture treatment for pain: systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups". BMJ. 338: a3115. doi:10.1136/bmj.a3115. PMC 2769056. PMID 19174438.
- Chiu HY, Hsieh YJ, Tsai PS (March 2017). "Systematic review and meta-analysis of acupuncture to reduce cancer-related pain". European Journal of Cancer Care. 26 (2): e12457. doi:10.1111/ecc.12457. PMID 26853524.
- Chang SC, Hsu CH, Hsu CK, Yang SS, Chang SJ (February 2017). "The efficacy of acupuncture in managing patients with chronic prostatitis/chronic pelvic pain syndrome: A systemic review and meta-analysis". Neurourology and Urodynamics. 36 (2): 474–481. doi:10.1002/nau.22958. PMID 26741647.
- Ji M, Wang X, Chen M, Shen Y, Zhang X, Yang J (2015). "The Efficacy of Acupuncture for the Treatment of Sciatica: A Systematic Review and Meta-Analysis". Evidence-Based Complementary and Alternative Medicine. 2015: 192808. doi:10.1155/2015/192808. PMC 4575738. PMID 26425130.
- Gagnier JJ, Oltean H, van Tulder MW, Berman BM, Bombardier C, Robbins CB (January 2016). "Herbal Medicine for Low Back Pain: A Cochrane Review". Spine. 41 (2): 116–33. doi:10.1097/BRS.0000000000001310. PMID 26630428.
- Straube S, Andrew Moore R, Derry S, McQuay HJ (January 2009). "Vitamin D and chronic pain". Pain. 141 (1–2): 10–3. doi:10.1016/j.pain.2008.11.010. PMID 19084336.
- Rubinstein, Sidney M.; Terwee, Caroline B.; Assendelft, Willem J. J.; de Boer, Michiel R.; van Tulder, Maurits W. (12 September 2012). "Spinal manipulative therapy for acute low-back pain". The Cochrane Database of Systematic Reviews (9): CD008880. doi:10.1002/14651858.CD008880.pub2. ISSN 1469-493X. PMC 6885055. PMID 22972127.
- Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ (May 2002). "The high prevalence of pain in emergency medical care". The American Journal of Emergency Medicine. 20 (3): 165–9. doi:10.1053/ajem.2002.32643. PMID 11992334.
- Hasselström J, Liu-Palmgren J, Rasjö-Wrååk G (2002). "Prevalence of pain in general practice". European Journal of Pain. 6 (5): 375–85. doi:10.1016/S1090-3801(02)00025-3. PMID 12160512.
- Abu-Saad Huijer H (2010). "Chronic pain: a review". Le Journal Medical Libanais. The Lebanese Medical Journal. 58 (1): 21–7. PMID 20358856.
- Smith AK, Cenzer IS, Knight SJ, Puntillo KA, Widera E, Williams BA, Boscardin WJ, Covinsky KE (November 2010). "The epidemiology of pain during the last 2 years of life". Annals of Internal Medicine. 153 (9): 563–9. doi:10.7326/0003-4819-153-9-201011020-00005. PMC 3150170. PMID 21041575.
- Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, van der Wouden JC (July 2000). "Pain in children and adolescents: a common experience". Pain. 87 (1): 51–8. doi:10.1016/S0304-3959(00)00269-4. PMID 10863045.
- Merskey H, Bogduk N (1994). Classification of Chronic Pain (2 nd ed.). Seattle: International Association for the Study of Pain. pp. 3 & 4. ISBN 978-0931092053.
- Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E (September 1998). "Towards a mechanism-based classification of pain?". Pain. 77 (3): 227–9. doi:10.1016/S0304-3959(98)00099-2. PMID 9808347.
- Woolf CJ (November 2010). "What is this thing called pain?". The Journal of Clinical Investigation. 120 (11): 3742–4. doi:10.1172/JCI45178. PMC 2965006. PMID 21041955.
- Rey R (1995). The history of pain. Cambridge: Harvard University Press. ISBN 9780674399686.
- Morris DR (1991). The culture of pain. Berkeley: University of California Press. ISBN 9780520082762.
- Backshall S (6 January 2008). "Bitten by the Amazon". The Sunday Times. London. Archived from the original on 22 February 2014.
- Working party of the Nuffield Council on Bioethics (2005). "The ethics of research involving animals. London: Nuffield Council on Bioethics." ISBN 9781904384106. Archived from the original on 25 June 2008. Retrieved 12 January 2010.
- Rollin BE (June 2007). "Animal research: a moral science. Talking Point on the use of animals in scientific research". EMBO Reports. 8 (6): 521–5. doi:10.1038/sj.embor.7400996. PMC 2002540. PMID 17545990.
- Rollin, B. (1989) The Unheeded Cry: Animal Consciousness, Animal Pain, and Science. New York: Oxford University Press, pp. xii, 117–118, cited in Carbone 2004, p. 150.
- Griffin DR, Speck GB (January 2004). "New evidence of animal consciousness". Animal Cognition. 7 (1): 5–18. doi:10.1007/s10071-003-0203-x. PMID 14658059.
- Allen C (January 1998). "Assessing animal cognition: ethological and philosophical perspectives". Journal of Animal Science. 76 (1): 42–7. doi:10.2527/1998.76142x. PMID 9464883.
- Sherwin, C.M., (2001). Can invertebrates suffer? Or, how robust is argument-by-analogy? Animal Welfare, 10 (supplement): S103-S118
- Lockwood JA (1987). "The Moral Standing of Insects and the Ethics of Extinction". The Florida Entomologist. 70 (1): 70–89. doi:10.2307/3495093. JSTOR 3495093.
- DeGrazia D, Rowan A (September 1991). "Pain, suffering, and anxiety in animals and humans". Theoretical Medicine. 12 (3): 193–211. doi:10.1007/BF00489606. PMID 1754965.
- Abbott FV, Franklin KB, Westbrook RF (January 1995). "The formalin test: scoring properties of the first and second phases of the pain response in rats". Pain. 60 (1): 91–102. doi:10.1016/0304-3959(94)00095-V. PMID 7715946.
- "Do Invertebrates Feel Pain?" Archived 6 January 2010 at the Wayback Machine, The Senate Standing Committee on Legal and Constitutional Affairs, The Parliament of Canada Web Site. Retrieved 11 June 2008.
- Smith JA (1991). "A Question of Pain in Invertebrates". Institute for Laboratory Animal Research Journal. 33: 1–2. Archived from the original on 8 October 2011.
- Eisemann CH, Jorgensen WK, Merritt DJ, Rice MJ, Cribb BW, Webb PD, Zalucki MP (1984). "Do insects feel pain? A biological view". Experientia. 40 (2): 164–167. doi:10.1007/BF01963580.
- Tracey WD, Wilson RI, Laurent G, Benzer S (April 2003). "painless, a Drosophila gene essential for nociception" (PDF). Cell. 113 (2): 261–73. doi:10.1016/S0092-8674(03)00272-1. PMID 12705873. Archived from the original (PDF) on 29 October 2005.
- Sukhdeo MV (1 January 1994). Parasites and Behaviour. Cambridge University Press. ISBN 9780521485425.
- L. Sømme (2005). "Sentience and pain in invertebrates: Report to Norwegian Scientific Committee for Food Safety". Norwegian University of Life Sciences, Oslo.
- Cephalopods and decapod crustaceans: their capacity to experience pain and suffering (PDF). Advocates for Animals. 2005. Archived from the original (PDF) on 6 April 2008.
- Viñuela-Fernández I, Jones E, Welsh EM, Fleetwood-Walker SM (September 2007). "Pain mechanisms and their implication for the management of pain in farm and companion animals". Veterinary Journal. 174 (2): 227–39. doi:10.1016/j.tvjl.2007.02.002. PMID 17553712.
- poena Archived 13 May 2011 at the Wayback Machine, Charlton T. Lewis, Charles Short, A Latin Dictionary, on Perseus Digital Library
- ποινή Archived 13 May 2011 at the Wayback Machine, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus Digital Library
- pain Archived 28 July 2011 at the Wayback Machine, Online Etymology Dictionary
External links
| Wikimedia Commons has media related to Pain. |
| Wikiquote has quotations related to: Pain |
| Classification | |
|---|---|
| External resources |