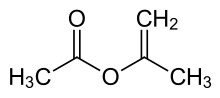
Isopropenyl acetate
Isopropenyl acetate is an organic compound, which is the acetate ester of the enol tautomer of acetone. This colorless liquid is significant commercially as the principal precursor to acetylacetone. In organic synthesis, it is used to prepare enol acetates of ketones and acetonides from diols.[1]
 | |
| Names | |
|---|---|
| Other names
1-Methylvinyl acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.239 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.117 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9090 g/cm3 (20 ºC) |
| Melting point | −92.9 °C (−135.2 °F; 180.2 K) |
| Boiling point | 97 °C (207 °F; 370 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
Isopropenyl acetate is prepared by treating acetone with ketene.[2] Upon heating over a metal surface, isopropenyl acetate rearranges to acetylacetone.[3]
- CH2(CH3)COC(O)Me → MeC(O)CH2C(O)Me
Isopropenyl acetate is used to prepare other isopropenyl esters by transesterification.[4]
gollark: New reddit is HERESY.
gollark: PCIe and DisplayPort and some weird power delivery stuff and likely more.
gollark: Plus Thunderbolt, being I think basically PCIe over an external cable, allows eGPUs and the like to work.
gollark: Oh, a notification LED sort of thing? Cool!
gollark: Oh, did the location of the ports get decided yet?
References
- Walters, Michael A.; Lee, Melissa D. (2001). "Isopropenyl Acetate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ri077.
- Miller, Raimund; Abaecherli, Claudio; Said, Adel; Jackson, Barry (2001). "Ketenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_063.
- Siegel, Hardo; Eggersdorfer, Manfred (2002). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.
- Obora, Yasushi; Ishii, Yasutaka (2012). "Discussion Addendum for: Iridium-catalyzed Synthesis of Vinyl Ethers from Alcohols and Vinyl Acetate". Org. Synth. 89: 307-310. doi:10.15227/orgsyn.089.0307.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.