Haematopoiesis
Haematopoiesis (/hɪˌmætoʊpɔɪˈiːsɪs, ˈhiːmətoʊ-, ˌhɛmə-/,[1] from Greek αἷμα, 'blood' and ποιεῖν 'to make'; also hematopoiesis in American English; sometimes also h(a)emopoiesis) is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells.[2] In a healthy adult person, approximately 1011–1012 new blood cells are produced daily in order to maintain steady state levels in the peripheral circulation.[3][4]
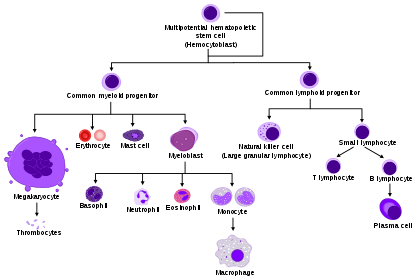
Process
Haematopoietic stem cells (HSCs)
Haematopoietic stem cells (HSCs) reside in the medulla of the bone (bone marrow) and have the unique ability to give rise to all of the different mature blood cell types and tissues.[2] HSCs are self-renewing cells: when they differentiate, at least some of their daughter cells remain as HSCs, so the pool of stem cells is not depleted. This phenomenon is called asymmetric division.[5] The other daughters of HSCs (myeloid and lymphoid progenitor cells) can follow any of the other differentiation pathways that lead to the production of one or more specific types of blood cell, but cannot renew themselves. The pool of progenitors is heterogeneous and can be divided into two groups; long-term self-renewing HSC and only transiently self-renewing HSC, also called short-terms.[6] This is one of the main vital processes in the body.
Cell types
All blood cells are divided into three lineages.[7]
- Red blood cells, also called erythrocytes, are the oxygen-carrying cells. Erythrocytes are functional and are released into the blood. The number of reticulocytes, immature red blood cells, gives an estimate of the rate of erythropoiesis.
- Lymphocytes are the cornerstone of the adaptive immune system. They are derived from common lymphoid progenitors. The lymphoid lineage is composed of T-cells, B-cells and natural killer cells. This is lymphopoiesis.
- Cells of the myeloid lineage, which include granulocytes, megakaryocytes and macrophages, are derived from common myeloid progenitors, and are involved in such diverse roles as innate immunity and blood clotting. This is myelopoiesis.
Granulopoiesis (or granulocytopoiesis) is haematopoiesis of granulocytes, except of mast cells which are granulocytes but with an extramedullar maturation.[8]
Megakaryocytopoiesis is haematopoiesis of megakaryocytes.
Terminology
Between 1948 and 1950, the Committee for Clarification of the Nomenclature of Cells and Diseases of the Blood and Blood-forming Organs issued reports on the nomenclature of blood cells.[9][10] An overview of the terminology is shown below, from earliest to final stage of development:
- [root]blast
- pro[root]cyte
- [root]cyte
- meta[root]cyte
- mature cell name
The root for erythrocyte colony-forming units (CFU-E) is "rubri", for granulocyte-monocyte colony-forming units (CFU-GM) is "granulo" or "myelo" and "mono", for lymphocyte colony-forming units (CFU-L) is "lympho" and for megakaryocyte colony-forming units (CFU-Meg) is "megakaryo". According to this terminology, the stages of red blood cell formation would be: rubriblast, prorubricyte, rubricyte, metarubricyte, and erythrocyte. However, the following nomenclature seems to be, at present, the most prevalent:
| Committee | "lympho" | "rubri" | "granulo" or "myelo" | "mono" | "megakaryo" |
|---|---|---|---|---|---|
| Lineage | Lymphoid | Myeloid | Myeloid | Myeloid | Myeloid |
| CFU | CFU-L | CFU-GEMM→CFU-E | CFU-GEMM→CFU-GM→CFU-G | CFU-GEMM→CFU-GM→CFU-M | CFU-GEMM→CFU-Meg |
| Process | lymphocytopoiesis | erythropoiesis | granulocytopoiesis | monocytopoiesis | thrombocytopoiesis |
| [root]blast | Lymphoblast | Proerythroblast | Myeloblast | Monoblast | Megakaryoblast |
| pro[root]cyte | Prolymphocyte | Polychromatophilic erythrocyte | Promyelocyte | Promonocyte | Promegakaryocyte |
| [root]cyte | – | Normoblast | Eosino/neutro/basophilic myelocyte | Megakaryocyte | |
| meta[root]cyte | Large lymphocyte | Reticulocyte | Eosinophilic/neutrophilic/basophilic metamyelocyte, Eosinophilic/neutrophilic/basophilic band cell | Early monocyte | - |
| mature cell name | Small lymphocyte | Erythrocyte | granulocytes (Eosino/neutro/basophil) | Monocyte | thrombocytes (Platelets) |
Osteoclasts also arise from hemopoietic cells of the monocyte/neutrophil lineage, specifically CFU-GM.
Location
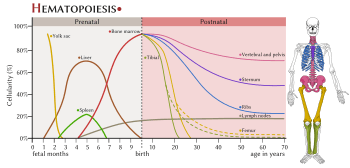
In developing embryos, blood formation occurs in aggregates of blood cells in the yolk sac, called blood islands. As development progresses, blood formation occurs in the spleen, liver and lymph nodes. When bone marrow develops, it eventually assumes the task of forming most of the blood cells for the entire organism.[2] However, maturation, activation, and some proliferation of lymphoid cells occurs in the spleen, thymus, and lymph nodes. In children, haematopoiesis occurs in the marrow of the long bones such as the femur and tibia. In adults, it occurs mainly in the pelvis, cranium, vertebrae, and sternum.[11]
Extramedullary
In some cases, the liver, thymus, and spleen may resume their haematopoietic function, if necessary. This is called extramedullary haematopoiesis. It may cause these organs to increase in size substantially. During fetal development, since bones and thus the bone marrow develop later, the liver functions as the main haematopoetic organ. Therefore, the liver is enlarged during development.[12] Extramedullary hematopoiesis and myelopoiesis may supply leukocytes in cardiovascular disease and inflammation during adulthood.[13][14] Splenic macrophages and adhesion molecules may be involved in regulation of extramedullary myeloid cell generation in cardiovascular disease.[15][16]
Maturation
_diagram_en.svg.png)
- The morphological characteristics of the hematopoietic cells are shown as seen in a Wright’s stain, May-Giemsa stain or May-Grünwald-Giemsa stain. Alternative names of certain cells are indicated between parentheses.
- Certain cells may have more than one characteristic appearance. In these cases, more than one representation of the same cell has been included.
- Together, the monocyte and the lymphocytes comprise the agranulocytes, as opposed to the granulocytes (basophil, neurtophil and eosinophil) that are produced during granulopoiesis.
- B., N. and E. stand for Basophilic, Neutrophilic and Eosinophilic, respectively – as in Basophilic promyelocyte. For lymphocytes, the T and B are actual designations.
- The polychromatic erythrocyte (reticulocyte) at the right shows its characteristic appearance when stained with methylene blue or Azure B.
- The erythrocyte at the right is a more accurate representation of its appearance in reality when viewed through a microscope.
- Other cells that arise from the monocyte: osteoclast, microglia (central nervous system), Langerhans cell (epidermis), Kupffer cell (liver).
- For clarity, the T and B lymphocyte are split to better indicate that the plasma cell arises from the B-cell. Note that there is no difference in the appearance of B- and T-cells unless specific staining is applied.
As a stem cell matures it undergoes changes in gene expression that limit the cell types that it can become and moves it closer to a specific cell type (cellular differentiation). These changes can often be tracked by monitoring the presence of proteins on the surface of the cell. Each successive change moves the cell closer to the final cell type and further limits its potential to become a different cell type.
Cell fate determination
Two models for hematopoiesis have been proposed: determinism and stochastic theory.[17] For the stem cells and other undifferentiated blood cells in the bone marrow, the determination is generally explained by the determinism theory of haematopoiesis, saying that colony stimulating factors and other factors of the haematopoietic microenvironment determine the cells to follow a certain path of cell differentiation.[2] This is the classical way of describing haematopoiesis. In stochastic theory, undifferentiated blood cells differentiate to specific cell types by randomness. This theory has been supported by experiments showing that within a population of mouse haematopoietic progenitor cells, underlying stochastic variability in the distribution of Sca-1, a stem cell factor, subdivides the population into groups exhibiting variable rates of cellular differentiation. For example, under the influence of erythropoietin (an erythrocyte-differentiation factor), a subpopulation of cells (as defined by the levels of Sca-1) differentiated into erythrocytes at a sevenfold higher rate than the rest of the population.[18] Furthermore, it was shown that if allowed to grow, this subpopulation re-established the original subpopulation of cells, supporting the theory that this is a stochastic, reversible process. Another level at which stochasticity may be important is in the process of apoptosis and self-renewal. In this case, the haematopoietic microenvironment prevails upon some of the cells to survive and some, on the other hand, to perform apoptosis and die.[2] By regulating this balance between different cell types, the bone marrow can alter the quantity of different cells to ultimately be produced.[19]
Growth factors
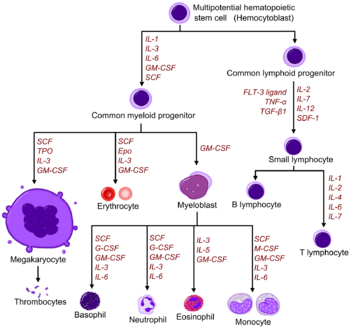
Red and white blood cell production is regulated with great precision in healthy humans, and the production of leukocytes is rapidly increased during infection. The proliferation and self-renewal of these cells depend on growth factors. One of the key players in self-renewal and development of haematopoietic cells is stem cell factor (SCF),[22] which binds to the c-kit receptor on the HSC. Absence of SCF is lethal. There are other important glycoprotein growth factors which regulate the proliferation and maturation, such as interleukins IL-2, IL-3, IL-6, IL-7. Other factors, termed colony-stimulating factors (CSFs), specifically stimulate the production of committed cells. Three CSFs are granulocyte-macrophage CSF (GM-CSF), granulocyte CSF (G-CSF) and macrophage CSF (M-CSF).[23] These stimulate granulocyte formation and are active on either progenitor cells or end product cells.
Erythropoietin is required for a myeloid progenitor cell to become an erythrocyte.[20] On the other hand, thrombopoietin makes myeloid progenitor cells differentiate to megakaryocytes (thrombocyte-forming cells).[20] The diagram to the right provides examples of cytokines and the differentiated blood cells they give rise to.[24]
Transcription factors
Growth factors initiate signal transduction pathways, which lead to activation of transcription factors. Growth factors elicit different outcomes depending on the combination of factors and the cell's stage of differentiation. For example, long-term expression of PU.1 results in myeloid commitment, and short-term induction of PU.1 activity leads to the formation of immature eosinophils.[25] Recently, it was reported that transcription factors such as NF-κB can be regulated by microRNAs (e.g., miR-125b) in haematopoiesis.[26]
The first key player of differentiation from HSC to a multipotent progenitor (MPP) is transcription factor CCAAT-enhancer binding protein α (C/EBPα). Mutations in C/EBPα are associated with acute myeloid leukaemia.[27] From this point, cells can either differentiate along the Erythroid-megakaryocyte lineage or lymphoid and myeloid lineage, which have common progenitor, called lymphoid-primed multipotent progenitor. There are two main transcription factors. PU.1 for Erythroid-megakaryocyte lineage and GATA-1, which leads to a lymphoid-primed multipotent progenitor.
Other transcription factors include Ikaros[28] (B cell development), and Gfi1[29] (promotes Th2 development and inhibits Th1) or IRF8[30] (basophils and mast cells). Significantly, certain factors elicit different responses at different stages in the haematopoiesis. For example, CEBPα in neutrophil development or PU.1 in monocytes and dendritic cell development. It is important to note that processes are not unidirectional: differentiated cells may regain attributes of progenitor cells.
An example is PAX5 factor, which is important in B cell development and associated with lymphomas.[31] Surprisingly, pax5 conditional knock out mice allowed peripheral mature B cells to de-differentiate to early bone marrow progenitors. These findings show that transcription factors act as caretakers of differentiation level and not only as initiators.[32]
Mutations in transcription factors are tightly connected to blood cancers, as acute myeloid leukaemia (AML) or acute lymphoblastic leukemia (ALL). For example, Ikaros is known to be regulator of numerous biological events. Mice with no Ikaros lack B cells, Natural killer and T cells.[33] Ikaros has six zinc fingers domains, four are conserved DNA-binding domain and two are for dimerization.[34] Very important finding is, that different zinc fingers are involved in binding to different place in DNA and this is the reason for pleiotropic effect of Ikaros and different involvement in cancer, but mainly are mutations associated with BCR-Abl patients and it is bad prognostic marker.[35]
Myeloid-based model
For a decade now, the evidence is growing that HSC maturation follows a myeloid-based model instead of the 'classical' schoolbook dichotomy model. In the latter model, the HSC first generates a common myeloid-erythroid progenitor (CMEP) and a common lymphoid progenitor (CLP). The CLP produces only T or B cells. The myeloid-based model postulates that HSCs first diverge into the CMEP and a common myelo-lymphoid progenitor (CMLP), which generates T and B cell progenitors through a bipotential myeloid-T progenitor and a myeloid-B progenitor stage. The main difference is that in this new model, all erythroid, T and B lineage branches retain the potential to generate myeloid cells (even after the segregation of T and B cell lineages). The model proposes the idea of erythroid, T and B cells as specialized types of a prototypic myeloid HSC.[36]
Other animals
In some vertebrates, haematopoiesis can occur wherever there is a loose stroma of connective tissue and slow blood supply, such as the gut, spleen or kidney.[37]
See also
- Clonal hematopoiesis
- Erythropoiesis-stimulating agents
- Haematon
- Haematopoietic stimulants:
- Granulocyte colony-stimulating factor
- Granulocyte macrophage colony-stimulating factor
- Leukocyte extravasation
References
- "haematopoiesis". Dictionary.com Unabridged. Random House. Retrieved 16 October 2019.
- Birbrair, Alexander; Frenette, Paul S. (1 March 2016). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370...82B. doi:10.1111/nyas.13016. ISSN 1749-6632. PMC 4938003. PMID 27015419.
- Semester 4 medical lectures at Uppsala University 2008 by Leif Jansson
- Parslow TG, Stites DP, Terr AI, Imboden JB. Medical Immunology (1 ed.). ISBN 978-0-8385-6278-9.
- Morrison, J.; Judith Kimble (2006). "Asymmetric and symmetric stem-cell divisions in development and cancer" (PDF). Nature. 441 (7097): 1068–74. Bibcode:2006Natur.441.1068M. doi:10.1038/nature04956. PMID 16810241.
- Morrison SJ, Weissman IL (November 1994). "The long-term repopulating subset of hematopoietic stem cells is deterministic and isolable by phenotype". Immunity. 1 (8): 661–73. doi:10.1016/1074-7613(94)90037-x. PMID 7541305.
- "Hematopoiesis from Pluripotent Stem Cells". Antibodies Resource Library. ThermoFisher Scientific. Retrieved 25 April 2020.
- Mahler (2013). Haschek, Wanda; Rousseaux, Colin G.; Wallig, Matthew A. (eds.). Haschek and Rousseaux's handbook of toxicologic pathology. associate editors, Brad Bolon and Ricardo Ochoa; illustrations editor, Beth W. (Third ed.). [S.l.]: Academic Press. p. 1863. ISBN 978-0-12-415759-0.
- "FIRST report of the committee for clarification of the nomenclature of cells and diseases of the blood and blood-forming organs". American Journal of Clinical Pathology. 18 (5): 443–50. May 1948. doi:10.1093/ajcp/18.5_ts.443. PMID 18913573.
- "THIRD, fourth and fifth reports of the committee for clarification of the nomenclature of cells and diseases of the blood and blood-forming organs". American Journal of Clinical Pathology. 20 (6): 562–79. June 1950. doi:10.1093/ajcp/20.6.562. PMID 15432355.
- Fernández KS, de Alarcón PA (December 2013). "Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood". Pediatric Clinics of North America. 60 (6): 1273–89. doi:10.1016/j.pcl.2013.08.002. PMID 24237971.
- Georgiades CS, Neyman EG, Francis IR, Sneider MB, Fishman EK (November 2002). "Typical and atypical presentations of extramedullary hemopoiesis". AJR. American Journal of Roentgenology. 179 (5): 1239–43. doi:10.2214/ajr.179.5.1791239. PMID 12388506.
- Swirski, Filip K.; Libby, Peter; Aikawa, Elena; Alcaide, Pilar; Luscinskas, F. William; Weissleder, Ralph; Pittet, Mikael J. (2 January 2007). "Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata". Journal of Clinical Investigation. 117 (1): 195–205. doi:10.1172/JCI29950. PMC 1716211. PMID 17200719.
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ (30 July 2009). "Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites". Science. 325 (5940): 612–616. Bibcode:2009Sci...325..612S. doi:10.1126/science.1175202. PMC 2803111. PMID 19644120.
- Dutta, P; Hoyer, FF; Grigoryeva, LS; Sager, HB; Leuschner, F; Courties, G; Borodovsky, A; Novobrantseva, T; Ruda, VM; Fitzgerald, K; Iwamoto, Y; Wojtkiewicz, G; Sun, Y; Da Silva, N; Libby, P; Anderson, DG; Swirski, FK; Weissleder, R; Nahrendorf, M (6 April 2015). "Macrophages retain hematopoietic stem cells in the spleen via VCAM-1". The Journal of Experimental Medicine. 212 (4): 497–512. doi:10.1084/jem.20141642. PMC 4387283. PMID 25800955.
- Dutta, P; Hoyer, FF; Sun, Y; Iwamoto, Y; Tricot, B; Weissleder, R; Magnani, JL; Swirski, FK; Nahrendorf, M (September 2016). "E-Selectin Inhibition Mitigates Splenic HSC Activation and Myelopoiesis in Hypercholesterolemic Mice With Myocardial Infarction". Arteriosclerosis, Thrombosis, and Vascular Biology. 36 (9): 1802–8. doi:10.1161/ATVBAHA.116.307519. PMC 5001901. PMID 27470513.
- Kimmel, Marek (1 January 2014). Stochasticity and determinism in models of hematopoiesis. Advances in Experimental Medicine and Biology. 844. pp. 119–152. doi:10.1007/978-1-4939-2095-2_7. ISBN 978-1-4939-2094-5. ISSN 0065-2598. PMID 25480640.
- Chang, Hannah H.; Hemberg, Martin; Barahona, Mauricio; Ingber, Donald E.; Huang, Sui (2008). "Transcriptome-wide noise controls lineage choice in mammalian progenitor cells". Nature. 453 (7194): 544–547. Bibcode:2008Natur.453..544C. doi:10.1038/nature06965. PMC 5546414. PMID 18497826.
- Alenzi, FQ; Alenazi, BQ; Ahmad, SY; Salem, ML; Al-Jabri, AA; Wyse, RK (March 2009). "The haemopoietic stem cell: between apoptosis and self renewal". The Yale Journal of Biology and Medicine. 82 (1): 7–18. PMC 2660591. PMID 19325941.
- Molecular cell biology. Lodish, Harvey F. 5. ed. : – New York : W. H. Freeman and Co., 2003, 973 s. b ill. ISBN 0-7167-4366-3
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000). "Cancers Originate in Proliferating Cells". Molecular Cell Biology (4th ed.). New York: W. H. Freeman. Figure 24-8: Formation of differentiated blood cells from hematopoietic stem cells in the bone marrow. ISBN 0-7167-3136-3 – via NCBI Bookshelf. - Rod Flower; Humphrey P. Rang; Maureen M. Dale; Ritter, James M. (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-06911-6.
- Broudy, VC (15 August 1997). "Stem cell factor and hematopoiesis". Blood. 90 (4): 1345–64. doi:10.1182/blood.V90.4.1345. PMID 9269751.
- Ketley, N. J.; A. C. Newland (1997). "Haemopoietic growth factors". Postgrad Med J. 73 (858): 215–221. doi:10.1136/pgmj.73.858.215. PMC 2431295. PMID 9156123.
- Hauke, Ralph; Stefano R. Tarantolo (November 2000). "Hematopoietic Growth Factors". Laboratory Medicine. 31 (11): 613–5. doi:10.1309/HNTM-ELUV-AV9G-MA1P.
- Engel, I; Murre, C (October 1999). "Transcription factors in hematopoiesis". Current Opinion in Genetics & Development. 9 (5): 575–9. doi:10.1016/s0959-437x(99)00008-8. PMID 10508690.
- O’Connell, R; Rao, D.; Baltimore, D (2012). "microRNA Regulation of Inflammatory Responses". Annual Review of Immunology. 30: 295–312. doi:10.1146/annurev-immunol-020711-075013. PMID 22224773.
- Ho, PA; Alonzo, TA; Gerbing, RB; Pollard, J; Stirewalt, DL; Hurwitz, C; Heerema, NA; Hirsch, B; Raimondi, SC; Lange, B; Franklin, JL; Radich, JP; Meshinchi, S (25 June 2009). "Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children's Oncology Group". Blood. 113 (26): 6558–66. doi:10.1182/blood-2008-10-184747. PMC 2943755. PMID 19304957.
- Thompson, Elizabeth C.; Cobb, Bradley S.; Sabbattini, Pierangela; Meixlsperger, Sonja; Parelho, Vania; Liberg, David; Taylor, Benjamin; Dillon, Niall; Georgopoulos, Katia (1 March 2007). "Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits". Immunity. 26 (3): 335–344. doi:10.1016/j.immuni.2007.02.010. ISSN 1074-7613. PMID 17363301.
- Suzuki, Junpei; Maruyama, Saho; Tamauchi, Hidekazu; Kuwahara, Makoto; Horiuchi, Mika; Mizuki, Masumi; Ochi, Mizuki; Sawasaki, Tatsuya; Zhu, Jinfang (1 April 2016). "Gfi1, a transcriptional repressor, inhibits the induction of the T helper type 1 programme in activated CD4 T cells". Immunology. 147 (4): 476–487. doi:10.1111/imm.12580. ISSN 1365-2567. PMC 4799889. PMID 26749286.
- Sasaki, Haruka; Kurotaki, Daisuke; Tamura, Tomohiko (1 April 2016). "Regulation of basophil and mast cell development by transcription factors". Allergology International. 65 (2): 127–134. doi:10.1016/j.alit.2016.01.006. ISSN 1440-1592. PMID 26972050.
- O'Brien, P; Morin, P, Jr; Ouellette, RJ; Robichaud, GA (15 December 2011). "The Pax-5 gene: a pluripotent regulator of B-cell differentiation and cancer disease". Cancer Research. 71 (24): 7345–50. doi:10.1158/0008-5472.CAN-11-1874. PMID 22127921.
- Cobaleda, C; Jochum, W; Busslinger, M (27 September 2007). "Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors". Nature. 449 (7161): 473–7. Bibcode:2007Natur.449..473C. doi:10.1038/nature06159. PMID 17851532.
- Wang, JH; Nichogiannopoulou, A; Wu, L; Sun, L; Sharpe, AH; Bigby, M; Georgopoulos, K (December 1996). "Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation". Immunity. 5 (6): 537–49. doi:10.1016/s1074-7613(00)80269-1. PMID 8986714.
- Sun, L; Liu, A; Georgopoulos, K (1 October 1996). "Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development". The EMBO Journal. 15 (19): 5358–69. doi:10.1002/j.1460-2075.1996.tb00920.x. PMC 452279. PMID 8895580.
- Schjerven, H; McLaughlin, J; Arenzana, TL; Frietze, S; Cheng, D; Wadsworth, SE; Lawson, GW; Bensinger, SJ; Farnham, PJ; Witte, ON; Smale, ST (October 2013). "Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros". Nature Immunology. 14 (10): 1073–83. doi:10.1038/ni.2707. PMC 3800053. PMID 24013668.
- Kawamoto, Hiroshi; Wada, Haruka Wada; Katsura, Yoshimoto (February 2010). "A revised scheme for developmental pathways of hematopoietic cells: the myeloid-based model". International Immunology. 22 (2): 65–70. doi:10.1093/intimm/dxp125. PMID 20053701.
- Zon, LI (15 October 1995). "Developmental biology of hematopoiesis". Blood (Review). 86 (8): 2876–91. doi:10.1182/blood.V86.8.2876.2876. PMID 7579378.
Further reading
- Godin, Isabelle; Cumano, Ana, eds. (2006). Hematopoietic stem cell development. Springer. ISBN 978-0-306-47872-7.