Chaperonin
Chaperonins are proteins that provide favourable conditions for the correct folding of other proteins, thus preventing aggregation. They prevent the misfolding of proteins, which prevents diseases such as Mad Cow Disease. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional tertiary structure. Chaperonins belong to a large class of molecules that assist protein folding, called molecular chaperones.[1][2] The energy to fold proteins is supplied by adenosine triphosphate (ATP). Chaperonin proteins may also tag misfolded proteins to be degraded.
Structure
The structure of these chaperonins resemble two donuts stacked on top of one another to create a barrel.
Each ring is composed of either 7, 8 or 9 subunits depending on the organism in which the chaperonin is found.
Categories of chaperonins
Group I
Group I chaperonins are found in bacteria as well as organelles of endosymbiotic origin: chloroplasts and mitochondria.
The GroEL/GroES complex in E. coli is a Group I chaperonin and the best characterized large (~ 1 MDa) chaperonin complex.
- GroEL is a double-ring 14mer with a greasy hydrophobic patch at its opening and can accommodate the native folding of substrates 15-60 kDa in size.
- GroES is a single-ring heptamer that binds to GroEL in the presence of ATP or transition state analogues of ATP hydrolysis, such as ADP-AlF3. It's like a cover that covers GroEL (box/bottle).
GroEL/GroES may not be able to undo protein aggregates, but kinetically it competes in the pathway of misfolding and aggregation, thereby preventing aggregate formation.[3]
Group II
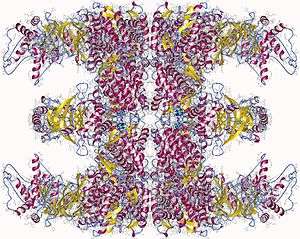
Group II chaperonins, found in the eukaryotic cytosol and in archaea, are more poorly characterized.
TRiC, the eukaryotic chaperonin, is composed of two rings of eight different though related subunits, each thought to be represented once per eight-membered ring. TRiC was originally thought to fold only the cytoskeletal proteins actin and tubulin but is now known to fold dozens of substrates.
Mm cpn (Methanococcus maripaludis chaperonin), found in the archaea Methanococcus maripaludis, is composed of sixteen identical subunits (eight per ring). It has been shown to fold the mitochondrial protein rhodanese; however, no natural substrates have yet been identified.[4]
Group II chaperonins are not thought to utilize a GroES-type cofactor to fold their substrates. They instead contain a "built-in" lid that closes in an ATP-dependent manner to encapsulate its substrates, a process that is required for optimal protein folding activity.
Mechanism of action
Chaperonins undergo large conformational changes during a folding reaction as a function of the enzymatic hydrolysis of ATP as well as binding of substrate proteins and cochaperonins, such as GroES. These conformational changes allow the chaperonin to bind an unfolded or misfolded protein, encapsulate that protein within one of the cavities formed by the two rings, and release the protein back into solution. Upon release, the substrate protein will either be folded or will require further rounds of folding, in which case it can again be bound by a chaperonin.
The exact mechanism by which chaperonins facilitate folding of substrate proteins is unknown. According to recent analyses by different experimental techniques, GroEL-bound substrate proteins populate an ensemble of compact and locally expanded states that lack stable tertiary interactions.[5] A number of models of chaperonin action have been proposed, which generally focus on two (not mutually exclusive) roles of chaperonin interior: passive and active. Passive models treat the chaperonin cage as an inert form, exerting influence by reducing the conformational space accessible to a protein substrate or preventing intermolecular interactions e.g. by aggregation prevention.[6] The active chaperonin role is in turn involved with specific chaperonin–substrate interactions that may be coupled to conformational rearrangements of the chaperonin.[7][8][9]
Probably the most popular model of the chaperonin active role is the iterative annealing mechanism (IAM), which focus on the effect of iterative, and hydrophobic in nature, binding of the protein substrate to the chaperonin. According to computational simulation studies, the IAM leads to more productive folding by unfolding the substrate from misfolded conformations[9] or by prevention from protein misfolding through changing the folding pathway.[7]
Conservation of structural and functional homology
As mentioned, all cells contain chaperonins.
- In bacteria, the archetype is the well-characterized chaperonin GroEL from E. coli.
- In archaea, the chaperonin is called the thermosome.
- In eukarya, the chaperonin is called CCT (also called TRiC).
These protein complexes appear to be essential for life in E. coli, Saccharomyces cerevisiae and higher eukaryotes. While there are differences between eukaryotic, bacterial and archaeal chaperonins, the general structure and mechanism are conserved.[2]
See also
References
- Howard Hughes Investigators: Arthur L. Horwich, M.D.
- Robb, Frank T.; Alberto J. L. Macario; Yohda, Masafumi; Macario, Everly Conway de (2019-03-15). "Bridging human chaperonopathies and microbial chaperonins". Communications Biology. 2 (1): 103. doi:10.1038/s42003-019-0318-5. ISSN 2399-3642. PMC 6420498. PMID 30911678.
- Fenton WA, Horwich AL (May 2003). "Chaperonin-mediated protein folding: fate of substrate polypeptide". Q. Rev. Biophys. 36 (2): 229–56. doi:10.1017/S0033583503003883. PMID 14686103.
- Kusmierczyk AR, Martin J (May 2003). "Nucleotide-dependent protein folding in the type II chaperonin from the mesophilic archaeon Methanococcus maripaludis". Biochem. J. 371 (3): 669–673. doi:10.1042/BJ20030230. PMC 1223359. PMID 12628000.
- Hartl, FU; Hayer-Hartl, M (2009). "Converging concepts of protein folding in vitro and in vivo". Nature Structural & Molecular Biology. 16 (6): 574–581. doi:10.1038/nsmb.1591. PMID 19491934.
- Apetri, AC; Horwich, AL (2008). "Chaperonin chamber accelerates protein folding through passive action of preventing aggregation". Proceedings of the National Academy of Sciences. 105 (45): 17351–17355. doi:10.1073/pnas.0809794105. PMC 2579888. PMID 18987317.
- Kmiecik, S; Kolinski, A (2011). "Simulation of Chaperonin Effect on Protein Folding: A Shift from Nucleation–Condensation to Framework Mechanism". Journal of the American Chemical Society. 133 (26): 10283–10289. doi:10.1021/ja203275f. PMC 3132998. PMID 21618995.
- Chakraborty, K; Chatila, M; Sinha, J; Shi, Q; Poschner, BC; Sikor, M; Jiang, G; Lamb, DC; Hartl, FU; Hayer-Hartl, M (2010). "Chaperonin-Catalyzed Rescue of Kinetically Trapped States in Protein Folding". Cell. 142 (1): 112–122. doi:10.1016/j.cell.2010.05.027. PMID 20603018.
- Todd, MJ; Lorimer, GH; Thirumalai, D. (1996). "Chaperonin-facilitated protein folding: optimization of rate and yield by an iterative annealing mechanism". Proceedings of the National Academy of Sciences. 93 (9): 4030–4035. doi:10.1073/pnas.93.9.4030. ISSN 0027-8424. PMC 39481. PMID 8633011.
External links
- more details...
- Chaperonins at the US National Library of Medicine Medical Subject Headings (MeSH)
- cpnDB: a chaperonin database
- Animations of activity of Chaperonins