Isoelectronicity
Isoelectronicity is an effect observed when distinct molecular entities have identical electron configurations. Two or more molecular entities are described as isoelectronic if they have the same number of valence electrons and the same structure, i.e. number and connectivity of atoms, but differ in some of the elements involved. [1]
This definition is sometimes termed valence isoelectronicity, in contrast with various alternatives. At one extreme these require identity of the total electron count and with it the entire electron configuration.[2] More usually, alternatives are broader, and may extend to allowing different numbers of atoms in the species being compared.[3]
The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic species can be expected to show useful consistency and predictability in their properties. (Slight differences of, for example, structural formula, such as a double versus single bond, commonly have major effects.)
Electron-density calculations have been performed on many common substances, resulting in reaction predictions. Identifying a new, rare or odd compound as isoelectronic with one already characterised offers clues to possible properties and reactions.
Examples
The N atom and the O+
radical ion are isoelectronic because each has five electrons in the outer electronic shell. Similarly, same number of valence electrons, same structure and same number of atoms the cations K+
, Ca2+
, and Sc3+
and the anions Cl−
, S2−
, and P3−
are all isoelectronic with the Ar atom. In such monatomic cases, there is a clear trend in the sizes of such species, with atomic radius decreasing as charge increases.
CO, CN−
, N
2, C2−
2, and NO+
are isoelectronic because each has two nuclei and 10 valence electrons, with each atom considered to have 5 of them (a lone-pair and a triple-bond). Isoelectronicity does not relate to formal charge on the atoms in a structure: these all have the same configuration even though carbon monoxide has formal charges that are balanced (−:C≡O:+) whereas dinitrogen has each atom neutral (:N≡N:) and nitrosonium has an overall net charge.
CO2, FCN, N2O, NO2+, N3−, NCO−, and CN22− are all isoelectronic, and each has multiple resonance forms: one with two double bonds and 2 lone pairs on each of the outer atoms, and one with one single bond and one triple bond.
Isoelectronicity leads to the concept of hydrogen-like atoms, ions with one electron which are thus isoelectronic with hydrogen.
The uncharged H
2C=C=O (ethenone) and H
2N-C≡N (cyanamide) molecules and the zwitterionic H
2C=N+
=N−
(diazomethane) molecule are isoelectronic.
CH
3COCH
3 (acetone) and CH
3N
2CH
3 (dimethyldiazene) are not isoelectronic. They do have the same number of nuclei and the same number of valence electrons, but the atoms' connectivity is different: the first one has both methyl (CH
3) groups attached to carbonyl's (CO's) carbon atom, forming a branched trigonal planar shape: H3C-C(=O)-CH3; the second molecule's structure has a consecutive attachment of the main atoms: H3C-N=N-CH3 and its methyl groups are not connected to the same nitrogen atom.
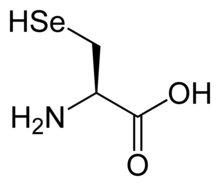
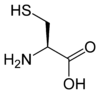
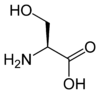
The amino acids tellurocysteine, selenocysteine, cysteine and serine are also considered (at least valence) isoelectronic.
See also
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isoelectronic". doi:10.1351/goldbook.I03276
- Isoelectronic Configurations iun.edu
- A A Aradi & T P Fehlner, 'Isoelectronic Organometallic Molecules', in F.G.A. Stone & Robert West (eds) Advances in Organometallic Chemistry Vol.30 (1990), Chapter 5 (at p.190) google books link