Transfer RNA
A transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA[1]) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length,[2] that serves as the physical link between the mRNA and the amino acid sequence of proteins. tRNA does this by carrying an amino acid to the protein synthetic machinery of a cell (ribosome) as directed by a 3-nucleotide sequence (codon) in a messenger RNA (mRNA). As such, tRNAs are a necessary component of translation, the biological synthesis of new proteins in accordance with the genetic code.
| tRNA | |
|---|---|
| Identifiers | |
| Symbol | t |
| Rfam | RF00005 |
| Other data | |
| RNA type | gene, tRNA |
| PDB structures | PDBe 3icq, 1asy, 1asz, 1il2, 2tra, 3tra, 486d, 1fir, 1yfg, 3eph, 3epj, 3epk, 3epl, 1efw, 1c0a, 2ake, 2azx, 2dr2, 1f7u, 1f7v, 3foz, 2hgp, 2j00 , 2j02, 2ow8, 2v46, 2v48, 2wdg, 2wdh, 2wdk, 2wdm, 2wh1 |
Overview
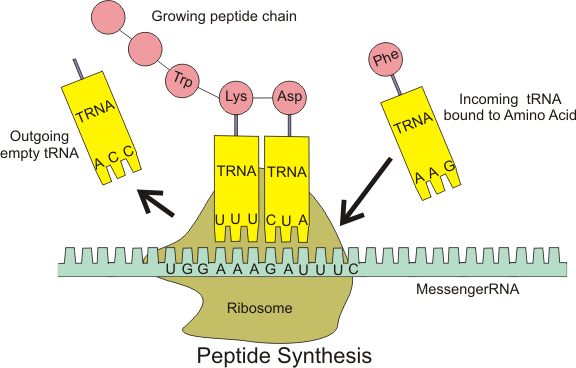
While the specific nucleotide sequence of an mRNA specifies which amino acids are incorporated into the protein product of the gene from which the mRNA is transcribed, the role of tRNA is to specify which sequence from the genetic code corresponds to which amino acid.[3] The mRNA encodes a protein as a series of contiguous codons, each of which is recognized by a particular tRNA. One end of the tRNA matches the genetic code in a three-nucleotide sequence called the anticodon. The anticodon forms three complementary base pairs with a codon in mRNA during protein biosynthesis. On the other end of the tRNA is a covalent attachment to the amino acid that corresponds to the anticodon sequence. Each type of tRNA molecule can be attached to only one type of amino acid, so each organism has many types of tRNA. Because the genetic code contains multiple codons that specify the same amino acid, there are several tRNA molecules bearing different anticodons which carry the same amino acid. The covalent attachment to the tRNA 3’ end is catalyzed by enzymes called aminoacyl tRNA synthetases. During protein synthesis, tRNAs with attached amino acids are delivered to the ribosome by proteins called elongation factors, which aid in association of the tRNA with the ribosome, synthesis of the new polypeptide, and translocation (movement) of the ribosome along the mRNA. If the tRNA's anticodon matches the mRNA, another tRNA already bound to the ribosome transfers the growing polypeptide chain from its 3’ end to the amino acid attached to the 3’ end of the newly delivered tRNA, a reaction catalyzed by the ribosome. A large number of the individual nucleotides in a tRNA molecule may be chemically modified, often by methylation or deamidation. These unusual bases sometimes affect the tRNA's interaction with ribosomes and sometimes occur in the anticodon to alter base-pairing properties.[4]
Structure
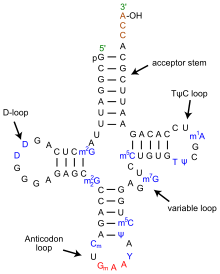
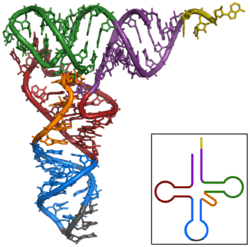
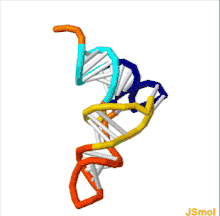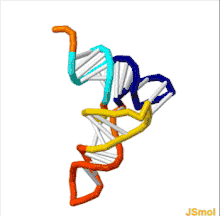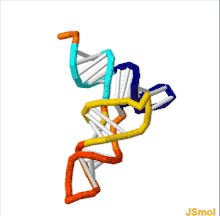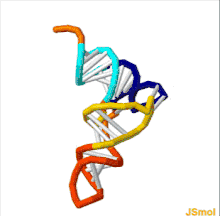
The structure of tRNA can be decomposed into its primary structure, its secondary structure (usually visualized as the cloverleaf structure), and its tertiary structure[6] (all tRNAs have a similar L-shaped 3D structure that allows them to fit into the P and A sites of the ribosome). The cloverleaf structure becomes the 3D L-shaped structure through coaxial stacking of the helices, which is a common RNA tertiary structure motif. The lengths of each arm, as well as the loop 'diameter', in a tRNA molecule vary from species to species.[6][7] The tRNA structure consists of the following:
- A 5'-terminal phosphate group.
- The acceptor stem is a 7- to 9-base pair (bp) stem made by the base pairing of the 5'-terminal nucleotide with the 3'-terminal nucleotide (which contains the CCA 3'-terminal group used to attach the amino acid). In general, such 3'-terminal tRNA-like structures are referred to as 'genomic tags'. The acceptor stem may contain non-Watson-Crick base pairs.[6][8]
- The CCA tail is a cytosine-cytosine-adenine sequence at the 3' end of the tRNA molecule. The amino acid loaded onto the tRNA by aminoacyl tRNA synthetases, to form aminoacyl-tRNA, is covalently bonded to the 3'-hydroxyl group on the CCA tail.[9] This sequence is important for the recognition of tRNA by enzymes and critical in translation.[10][11] In prokaryotes, the CCA sequence is transcribed in some tRNA sequences. In most prokaryotic tRNAs and eukaryotic tRNAs, the CCA sequence is added during processing and therefore does not appear in the tRNA gene.[12]
- The D arm is a 4- to 6-bp stem ending in a loop that often contains dihydrouridine.[6]
- The anticodon arm is a 5-bp stem whose loop contains the anticodon.[6] The tRNA 5'-to-3' primary structure contains the anticodon but in reverse order, since 3'-to-5' directionality is required to read the mRNA from 5'-to-3'.
- The T arm is a 4- to 5- bp stem containing the sequence TΨC where Ψ is pseudouridine, a modified uridine.[6]
- Bases that have been modified, especially by methylation (e.g. tRNA (guanine-N7-)-methyltransferase), occur in several positions throughout the tRNA. The first anticodon base, or wobble-position, is sometimes modified to inosine (derived from adenine), queuosine (derived from guanine), uridine-5-oxyacetic acid (derived from uracil), 5-methylaminomethyl-2-thiouridine (derived from uracil), or lysidine (derived from cytosine).[13]
Anticodon
An anticodon[14] is a unit of three nucleotides corresponding to the three bases of an mRNA codon. Each tRNA has a distinct anticodon triplet sequence that can form 3 complementary base pairs to one or more codons for an amino acid. Some anticodons pair with more than one codon due to wobble base pairing. Frequently, the first nucleotide of the anticodon is one not found on mRNA: inosine, which can hydrogen bond to more than one base in the corresponding codon position.[4]:29.3.9 In genetic code, it is common for a single amino acid to be specified by all four third-position possibilities, or at least by both pyrimidines and purines; for example, the amino acid glycine is coded for by the codon sequences GGU, GGC, GGA, and GGG. Other modified nucleotides may also appear at the first anticodon position—sometimes known as the "wobble position"—resulting in subtle changes to the genetic code, as for example in mitochondria.[15] Per cell, 61 tRNA types are required to provide one-to-one correspondence between tRNA molecules and codons that specify amino acids, as there are 61 sense codons of the standard genetic code. However, many cells have under 61 types of tRNAs because the wobble base is capable of binding to several, though not necessarily all, of the codons that specify a particular amino acid. At least 31 tRNAs are required to translate, unambiguously, all 61 sense codons; the maximum observed is 41.[3][16]
Aminoacylation
Aminoacylation is the process of adding an aminoacyl group to a compound. It covalently links an amino acid to the CCA 3' end of a tRNA molecule. Each tRNA is aminoacylated (or charged) with a specific amino acid by an aminoacyl tRNA synthetase. There is normally a single aminoacyl tRNA synthetase for each amino acid, despite the fact that there can be more than one tRNA, and more than one anticodon for an amino acid. Recognition of the appropriate tRNA by the synthetases is not mediated solely by the anticodon, and the acceptor stem often plays a prominent role.[17] Reaction:
Certain organisms can have one or more aminoacyl tRNA synthetases missing. This leads to charging of the tRNA by a chemically related amino acid, and by use of an enzyme or enzymes, the tRNA is modified to be correctly charged. For example, Helicobacter pylori has glutaminyl tRNA synthetase missing. Thus, glutamate tRNA synthetase charges tRNA-glutamine(tRNA-Gln) with glutamate. An amidotransferase then converts the acid side chain of the glutamate to the amide, forming the correctly charged gln-tRNA-Gln.
Binding to ribosome
The ribosome has three binding sites for tRNA molecules that span the space between the two ribosomal subunits: the A (aminoacyl),[19] P (peptidyl), and E (exit) sites. In addition, the ribosome has two other sites for tRNA binding that are used during mRNA decoding or during the initiation of protein synthesis. These are the T site (named elongation factor Tu) and I site (initiation).[20][21] By convention, the tRNA binding sites are denoted with the site on the small ribosomal subunit listed first and the site on the large ribosomal subunit listed second. For example, the A site is often written A/A, the P site, P/P, and the E site, E/E.[20] The binding proteins like L27, L2, L14, L15, L16 at the A- and P- sites have been determined by affinity labeling by A.P. Czernilofsky et al. (Proc. Natl. Acad. Sci, USA, pp. 230–34, 1974).
Once translation initiation is complete, the first aminoacyl tRNA is located in the P/P site, ready for the elongation cycle described below. During translation elongation, tRNA first binds to the ribosome as part of a complex with elongation factor Tu (EF-Tu) or its eukaryotic (eEF-1) or archaeal counterpart. This initial tRNA binding site is called the A/T site. In the A/T site, the A-site half resides in the small ribosomal subunit where the mRNA decoding site is located. The mRNA decoding site is where the mRNA codon is read out during translation. The T-site half resides mainly on the large ribosomal subunit where EF-Tu or eEF-1 interacts with the ribosome. Once mRNA decoding is complete, the aminoacyl-tRNA is bound in the A/A site and is ready for the next peptide bond to be formed to its attached amino acid. The peptidyl-tRNA, which transfers the growing polypeptide to the aminoacyl-tRNA bound in the A/A site, is bound in the P/P site. Once the peptide bond is formed, the tRNA in the P/P site is deacylated, or has a free 3’ end, and the tRNA in the A/A site carries the growing polypeptide chain. To allow for the next elongation cycle, the tRNAs then move through hybrid A/P and P/E binding sites, before completing the cycle and residing in the P/P and E/E sites. Once the A/A and P/P tRNAs have moved to the P/P and E/E sites, the mRNA has also moved over by one codon and the A/T site is vacant, ready for the next round of mRNA decoding. The tRNA bound in the E/E site then leaves the ribosome.
The P/I site is actually the first to bind to aminoacyl tRNA, which is delivered by an initiation factor called IF2 in bacteria.[21] However, the existence of the P/I site in eukaryotic or archaeal ribosomes has not yet been confirmed. The P-site protein L27 has been determined by affinity labeling by E. Collatz and A.P. Czernilofsky (FEBS Lett., Vol. 63, pp. 283–86, 1976).
tRNA genes
Organisms vary in the number of tRNA genes in their genome. For example, the nematode worm C. elegans, a commonly used model organism in genetics studies, has 29,647 [22] genes in its nuclear genome, of which 620 code for tRNA.[23][24] The budding yeast Saccharomyces cerevisiae has 275 tRNA genes in its genome.
In the human genome, which, according to January 2013 estimates, has about 20,848 protein coding genes [25] in total, there are 497 nuclear genes encoding cytoplasmic tRNA molecules, and 324 tRNA-derived pseudogenes—tRNA genes thought to be no longer functional[26] (although pseudo tRNAs have been shown to be involved in antibiotic resistance in bacteria ).[27] Regions in nuclear chromosomes, very similar in sequence to mitochondrial tRNA genes, have also been identified (tRNA-lookalikes).[28] These tRNA-lookalikes are also considered part of the nuclear mitochondrial DNA (genes transferred from the mitochondria to the nucleus).[28][29]
As with all eukaryotes, there are 22 mitochondrial tRNA genes[30] in humans. Mutations in some of these genes have been associated with severe diseases like the MELAS syndrome.
Cytoplasmic tRNA genes can be grouped into 49 families according to their anticodon features. These genes are found on all chromosomes, except the 22 and Y chromosome. High clustering on 6p is observed (140 tRNA genes), as well on 1 chromosome.[26]
The HGNC, in collaboration with the Genomic tRNA Database (GtRNAdb) and experts in the field, has approved unique names for human genes that encode tRNAs.
Evolution
The top half of tRNA (consisting of the T arm and the acceptor stem with 5'-terminal phosphate group and 3'-terminal CCA group) and the bottom half (consisting of the D arm and the anticodon arm) are independent units in structure as well as in function. The top half may have evolved first including the 3'-terminal genomic tag which originally may have marked tRNA-like molecules for replication in early RNA world. The bottom half may have evolved later as an expansion, e.g. as protein synthesis started in RNA world and turned it into a ribonucleoprotein world (RNP world). This proposed scenario is called genomic tag hypothesis. In fact, tRNA and tRNA-like aggregates have an important catalytic influence (i. e. as ribozymes) on replication still today. These roles may be regarded as 'molecular (or chemical) fossils' of RNA world.[31]
Genomic tRNA content is a differentiating feature of genomes among biological domains of life: Archaea present the simplest situation in terms of genomic tRNA content with a uniform number of gene copies, Bacteria have an intermediate situation and Eukarya present the most complex situation.[32] Eukarya present not only more tRNA gene content than the other two kingdoms but also a high variation in gene copy number among different isoacceptors, and this complexity seem to be due to duplications of tRNA genes and changes in anticodon specificity .
Evolution of the tRNA gene copy number across different species has been linked to the appearance of specific tRNA modification enzymes (uridine methyltransferases in Bacteria, and adenosine deaminases in Eukarya), which increase the decoding capacity of a given tRNA.[32] As an example, tRNAAla encodes four different tRNA isoacceptors (AGC, UGC, GGC and CGC). In Eukarya, AGC isoacceptors are extremely enriched in gene copy number in comparison to the rest of isoacceptors, and this has been correlated with its A-to-I modification of its wobble base. This same trend has been shown for most amino acids of eukaryal species. Indeed, the effect of these two tRNA modifications is also seen in codon usage bias. Highly expressed genes seem to be enriched in codons that are exclusively using codons that will be decoded by these modified tRNAs, which suggests a possible role of these codons—and consequently of these tRNA modifications—in translation efficiency.[32]
tRNA-derived fragments
tRNA-derived fragments (or tRFs) are short molecules that emerge after cleavage of the mature tRNAs or the precursor transcript.[33][34][35][36] Both cytoplasmic and mitochondrial tRNAs can produce fragments. There are at least four structural types of tRFs believed to originate from mature tRNAs, including the relatively long tRNA halves and short 5’-tRFs, 3’-tRFs and i-tRFs.[33][37] The precursor tRNA can be cleaved to produce molecules from the 5’ leader or 3’ trail sequences. Cleavage enzymes include Angiogenin, Dicer, RNase Z and RNase P.[33][34] Especially in the case of Angiogenin, the tRFs have a characteristically unusual cyclic phosphate at their 3’ end and a hydroxyl group at the 5’ end.[38] tRFs appear to play a role in RNA interference, specifically in the suppression of retroviruses and retrotransposons that use tRNA as a primer for replication. Half-tRNAs cleaved by angiogenin are also known as tiRNAs. The biogensis of smaller fragments, including those that function as piRNAs, are less understood.[39]
tRFs have multiple dependencies and roles; such as exhibiting significant changes between sexes, among races and disease status. Functionally, they can be loaded on Ago and act through RNAi pathways,[35][37][40] participate in the formation of stress granules,[41] displace mRNAs from RNA-binding proteins[42] or inhibit translation.[43] At the system or the organismal level, the four types of tRFs have a diverse spectrum of activities. Functionally, tRFs are associated with viral infection,[44] cancer,[37] cell proliferation [38] and also with epigenetic transgenerational regulation of metabolism.[45]
tRFs are not restricted to humans and have been shown to exist in multiple organisms.[37][46][47][48]
Two online tools are available for those wishing to learn more about tRFs: the framework for the interactive exploration of mitochondrial and nuclear tRNA fragments (MINTbase)[49] and the relational database of Transfer RNA related Fragments (tRFdb).[50] MINTbase also provides a naming scheme for the naming of tRFs called tRF-license plates (or MINTcodes) that is genome independent; the scheme compresses an RNA sequence into a shorter string.
Engineered tRNAs
Artificial suppressor elongator tRNAs are used to incorporate unnatural amino acids at nonsense codons placed in the coding sequence of a gene. Engineered initiator tRNAs (tRNAfMet2 with CUA anticodon encoded by metY gene) have been used to initiate translation at the amber stop codon UAG. This type of engineered tRNA is called a nonsense suppressor tRNA because it suppresses the translation stop signal that normally occurs at UAG codons. The amber initiator tRNA inserts methionine[51] and glutamine[52] at UAG codons preceded by a strong Shine-Dalgarno sequence. An investigation of the amber initiator tRNA showed that it was orthogonal to the regular AUG start codon showing no detectable off-target translation initiation events in a genomically recoded E. coli strain.[51]
tRNA biogenesis
In eukaryotic cells, tRNAs are transcribed by RNA polymerase III as pre-tRNAs in the nucleus.[53] RNA polymerase III recognizes two highly conserved downstream promoter sequences: the 5' intragenic control region (5'-ICR, D-control region, or A box), and the 3'-ICR (T-control region or B box) inside tRNA genes.[2][54][55] The first promoter begins at +8 of mature tRNAs and the second promoter is located 30–60 nucleotides downstream of the first promoter. The transcription terminates after a stretch of four or more thymidines.[2][55]
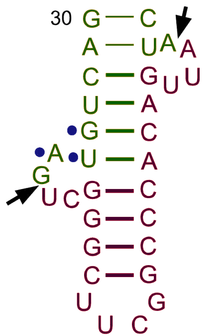
Pre-tRNAs undergo extensive modifications inside the nucleus. Some pre-tRNAs contain introns that are spliced, or cut, to form the functional tRNA molecule;[56] in bacteria these self-splice, whereas in eukaryotes and archaea they are removed by tRNA-splicing endonucleases.[57] Eukaryotic pre-tRNA contains bulge-helix-bulge (BHB) structure motif that is important for recognition and precise splicing of tRNA intron by endonucleases.[58] This motif position and structure are evolutionarily conserved. However, some organisms, such as unicellular algae have a non-canonical position of BHB-motif as well as 5'- and 3'-ends of the spliced intron sequence.[58] The 5' sequence is removed by RNase P,[59] whereas the 3' end is removed by the tRNase Z enzyme.[60] A notable exception is in the archaeon Nanoarchaeum equitans, which does not possess an RNase P enzyme and has a promoter placed such that transcription starts at the 5' end of the mature tRNA.[61] The non-templated 3' CCA tail is added by a nucleotidyl transferase.[62] Before tRNAs are exported into the cytoplasm by Los1/Xpo-t,[63][64] tRNAs are aminoacylated.[65] The order of the processing events is not conserved. For example, in yeast, the splicing is not carried out in the nucleus but at the cytoplasmic side of mitochondrial membranes.[66]
History
The existence of tRNA was first hypothesized by Francis Crick, based on the assumption that there must exist an adapter molecule capable of mediating the translation of the RNA alphabet into the protein alphabet. Paul C Zamecnik and Mahlon Hoagland discovered tRNA [67] Significant research on structure was conducted in the early 1960s by Alex Rich and Don Caspar, two researchers in Boston, the Jacques Fresco group in Princeton University and a United Kingdom group at King's College London.[68] In 1965, Robert W. Holley of Cornell University reported the primary structure and suggested three secondary structures.[69] tRNA was first crystallized in Madison, Wisconsin, by Robert M. Bock.[70] The cloverleaf structure was ascertained by several other studies in the following years[71] and was finally confirmed using X-ray crystallography studies in 1974. Two independent groups, Kim Sung-Hou working under Alexander Rich and a British group headed by Aaron Klug, published the same crystallography findings within a year.[72][73]
See also
- Cloverleaf model of tRNA
- Kim Sung-Hou
- Kissing stem-loop
- mRNA
- non-coding RNA and introns
- Slippery sequence
- tmRNA
- Transfer RNA-like structures
- Translation
- tRNADB
- Wobble hypothesis
- Aminoacyl-tRNA
References
- Plescia OJ, Palczuk NC, Cora-Figueroa E, Mukherjee A, Braun W (October 1965). "Production of antibodies to soluble RNA (sRNA)". Proceedings of the National Academy of Sciences of the United States of America. 54 (4): 1281–5. Bibcode:1965PNAS...54.1281P. doi:10.1073/pnas.54.4.1281. PMC 219862. PMID 5219832.
- Sharp SJ, Schaack J, Cooley L, Burke DJ, Söll D (1985). "Structure and transcription of eukaryotic tRNA genes". CRC Critical Reviews in Biochemistry. 19 (2): 107–44. doi:10.3109/10409238509082541. PMID 3905254.
- Crick FH (December 1968). "The origin of the genetic code". Journal of Molecular Biology. 38 (3): 367–79. doi:10.1016/0022-2836(68)90392-6. PMID 4887876.
- Stryer L, Berg JM, Tymoczko JL (2002). Biochemistry (5th ed.). San Francisco: W.H. Freeman. ISBN 978-0-7167-4955-4.
- "Transfer RNA (tRNA)". Proteopedia.org. Retrieved 7 November 2018.
- Itoh Y, Sekine S, Suetsugu S, Yokoyama S (July 2013). "Tertiary structure of bacterial selenocysteine tRNA". Nucleic Acids Research. 41 (13): 6729–38. doi:10.1093/nar/gkt321. PMC 3711452. PMID 23649835.
- Goodenbour JM, Pan T (29 October 2006). "Diversity of tRNA genes in eukaryotes". Nucleic Acids Research. 34 (21): 6137–46. doi:10.1093/nar/gkl725. PMC 1693877. PMID 17088292.
- Jahn M, Rogers MJ, Söll D (July 1991). "Anticodon and acceptor stem nucleotides in tRNA(Gln) are major recognition elements for E. coli glutaminyl-tRNA synthetase". Nature. 352 (6332): 258–60. Bibcode:1991Natur.352..258J. doi:10.1038/352258a0. PMID 1857423.
- Ibba M, Soll D (June 2000). "Aminoacyl-tRNA synthesis". Annual Review of Biochemistry. 69 (1): 617–50. doi:10.1146/annurev.biochem.69.1.617. PMID 10966471.
- Sprinzl M, Cramer F (1979). "The -C-C-A end of tRNA and its role in protein biosynthesis". Progress in Nucleic Acid Research and Molecular Biology. 22: 1–69. doi:10.1016/s0079-6603(08)60798-9. ISBN 9780125400220. PMID 392600.
- Green R, Noller HF (1997). "Ribosomes and translation". Annual Review of Biochemistry. 66: 679–716. doi:10.1146/annurev.biochem.66.1.679. PMID 9242921.
- Aebi M, Kirchner G, Chen JY, Vijayraghavan U, Jacobson A, Martin NC, Abelson J, et al. (September 1990). "Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae". The Journal of Biological Chemistry. 265 (27): 16216–20. PMID 2204621.
- McCloskey JA, Nishimura S (November 1977). "Modified nucleosides in transfer RNA". Accounts of Chemical Research. 10 (11): 403–10. doi:10.1021/ar50119a004.
- Felsenfeld G, Cantoni GL (May 1964). "Use of thermal denaturation studies to investigate the base sequence of yeast serine sRNA". Proceedings of the National Academy of Sciences of the United States of America. 51 (5): 818–26. Bibcode:1964PNAS...51..818F. doi:10.1073/pnas.51.5.818. PMC 300168. PMID 14172997.
- Suzuki T, Suzuki T (June 2014). "A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs". Nucleic Acids Research. 42 (11): 7346–57. doi:10.1093/nar/gku390. PMC 4066797. PMID 24831542.
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. (2004). Molecular Biology of the Cell. WH Freeman: New York. 5th ed.
- Schimmel P, Giegé R, Moras D, Yokoyama S (October 1993). "An operational RNA code for amino acids and possible relationship to genetic code". Proceedings of the National Academy of Sciences of the United States of America. 90 (19): 8763–8. Bibcode:1993PNAS...90.8763S. doi:10.1073/pnas.90.19.8763. PMC 47440. PMID 7692438.
- Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH (May 2011). "Structures of the bacterial ribosome in classical and hybrid states of tRNA binding". Science. 332 (6032): 981–4. Bibcode:2011Sci...332..981D. doi:10.1126/science.1202692. PMC 3176341. PMID 21596992.
- Konevega AL, Soboleva NG, Makhno VI, Semenkov YP, Wintermeyer W, Rodnina MV, Katunin VI (January 2004). "Purine bases at position 37 of tRNA stabilize codon-anticodon interaction in the ribosomal A site by stacking and Mg2+-dependent interactions". RNA. 10 (1): 90–101. doi:10.1261/rna.5142404. PMC 1370521. PMID 14681588.
- Agirrezabala X, Frank J (August 2009). "Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu". Quarterly Reviews of Biophysics. 42 (3): 159–200. doi:10.1017/S0033583509990060. PMC 2832932. PMID 20025795.
- Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J (June 2005). "The cryo-EM structure of a translation initiation complex from Escherichia coli". Cell. 121 (5): 703–12. doi:10.1016/j.cell.2005.03.023. PMID 15935757.
- WormBase web site, http://www.wormbase.org, release WS187, date 25-Jan-2008.
- Spieth J, Lawson D (January 2006). "Overview of gene structure". WormBook: 1–10. doi:10.1895/wormbook.1.65.1. PMC 4781370. PMID 18023127.
- Hartwell LH, Hood L, Goldberg ML, Reynolds AE, Silver LM, Veres RC. (2004). Genetics: From Genes to Genomes 2nd ed. McGraw-Hill: New York. p. 264.
- Ensembl release 70 - Jan 2013 http://www.ensembl.org/Homo_sapiens/Info/StatsTable?db=core Archived 2013-12-15 at the Wayback Machine
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. (International Human Genome Sequencing Consortium) (February 2001). "Initial sequencing and analysis of the human genome" (PDF). Nature. 409 (6822): 860–921. Bibcode:2001Natur.409..860L. doi:10.1038/35057062. PMID 11237011.
- Rogers TE, Ataide SF, Dare K, Katz A, Seveau S, Roy H, Ibba M (2012). "A pseudo-tRNA modulates antibiotic resistance in Bacillus cereus". PLOS One. 7 (7): e41248. Bibcode:2012PLoSO...741248R. doi:10.1371/journal.pone.0041248. PMC 3399842. PMID 22815980.
- Telonis AG, Loher P, Kirino Y, Rigoutsos I (2014). "Nuclear and mitochondrial tRNA-lookalikes in the human genome". Frontiers in Genetics. 5: 344. doi:10.3389/fgene.2014.00344. PMC 4189335. PMID 25339973.
- Ramos A, Barbena E, Mateiu L, del Mar González M, Mairal Q, Lima M, Montiel R, Aluja MP, Santos C, et al. (November 2011). "Nuclear insertions of mitochondrial origin: Database updating and usefulness in cancer studies". Mitochondrion. 11 (6): 946–53. doi:10.1016/j.mito.2011.08.009. PMID 21907832.
- Ibid. p. 529.
- Nancy Maizels and Alan M. Weiner: The Genomic Tag Hypothesis – What Molecular Fossils Tell Us about the Evolution of tRNA, in: The RNA World, Second Edition. 1999 Cold Spring Harbor Laboratory Press ISBN 0-87969-561-7/99, PDF
- Novoa EM, Pavon-Eternod M, Pan T, Ribas de Pouplana L (March 2012). "A role for tRNA modifications in genome structure and codon usage". Cell. 149 (1): 202–13. doi:10.1016/j.cell.2012.01.050. PMID 22464330.
- Gebetsberger J, Polacek N (December 2013). "Slicing tRNAs to boost functional ncRNA diversity". RNA Biology. 10 (12): 1798–806. doi:10.4161/rna.27177. PMC 3917982. PMID 24351723.
- Shigematsu M, Honda S, Kirino Y (2014). "Transfer RNA as a source of small functional RNA". Journal of Molecular Biology and Molecular Imaging. 1 (2): 8. PMC 4572697. PMID 26389128.
- Sobala A, Hutvagner G (2011). "Transfer RNA-derived fragments: origins, processing, and functions" (PDF). Wiley Interdisciplinary Reviews: RNA. 2 (6): 853–62. doi:10.1002/wrna.96. hdl:10453/18187. PMID 21976287.
- Keam SP, Hutvagner G (November 2015). "tRNA-Derived Fragments (tRFs): Emerging New Roles for an Ancient RNA in the Regulation of Gene Expression". Life. 5 (4): 1638–51. doi:10.3390/life5041638. PMC 4695841. PMID 26703738.
- Kumar P, Anaya J, Mudunuri SB, Dutta A (October 2014). "Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets". BMC Biology. 12: 78. doi:10.1186/s12915-014-0078-0. PMC 4203973. PMID 25270025.
- Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, Kirino Y (July 2015). "Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers". Proceedings of the National Academy of Sciences of the United States of America. 112 (29): E3816-25. Bibcode:2015PNAS..112E3816H. doi:10.1073/pnas.1510077112. PMC 4517238. PMID 26124144.
- Schorn, AJ; Martienssen, R (October 2018). "Tie-Break: Host and Retrotransposons Play tRNA". Trends in Cell Biology. 28 (10): 793–806. doi:10.1016/j.tcb.2018.05.006. PMC 6520983. PMID 29934075.
- Shigematsu M, Kirino Y (2015). "tRNA-Derived Short Non-coding RNA as Interacting Partners of Argonaute Proteins". Gene Regulation and Systems Biology. 9: 27–33. doi:10.4137/GRSB.S29411. PMC 4567038. PMID 26401098.
- Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P (April 2010). "Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly". The Journal of Biological Chemistry. 285 (14): 10959–68. doi:10.1074/jbc.M109.077560. PMC 2856301. PMID 20129916.
- Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF (May 2015). "Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement". Cell. 161 (4): 790–802. doi:10.1016/j.cell.2015.02.053. PMC 4457382. PMID 25957686.
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P (August 2011). "Angiogenin-induced tRNA fragments inhibit translation initiation". Molecular Cell. 43 (4): 613–23. doi:10.1016/j.molcel.2011.06.022. PMC 3160621. PMID 21855800.
- Selitsky SR, Baran-Gale J, Honda M, Yamane D, Masaki T, Fannin EE, Guerra B, Shirasaki T, Shimakami T, Kaneko S, Lanford RE, Lemon SM, Sethupathy P (January 2015). "Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C". Scientific Reports. 5: 7675. Bibcode:2015NatSR...5E7675S. doi:10.1038/srep07675. PMC 4286764. PMID 25567797.
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, Song L, Carone BR, Ricci EP, Li XZ, Fauquier L, Moore MJ, Sullivan R, Mello CC, Garber M, Rando OJ (January 2016). "Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals". Science. 351 (6271): 391–396. Bibcode:2016Sci...351..391S. doi:10.1126/science.aad6780. PMC 4888079. PMID 26721685.
- Casas E, Cai G, Neill JD (2015). "Characterization of circulating transfer RNA-derived RNA fragments in cattle". Frontiers in Genetics. 6: 271. doi:10.3389/fgene.2015.00271. PMC 4547532. PMID 26379699.
- Hirose Y, Ikeda KT, Noro E, Hiraoka K, Tomita M, Kanai A (July 2015). "Precise mapping and dynamics of tRNA-derived fragments (tRFs) in the development of Triops cancriformis (tadpole shrimp)". BMC Genetics. 16: 83. doi:10.1186/s12863-015-0245-5. PMC 4501094. PMID 26168920.
- Karaiskos S, Naqvi AS, Swanson KE, Grigoriev A (September 2015). "Age-driven modulation of tRNA-derived fragments in Drosophila and their potential targets". Biology Direct. 10: 51. doi:10.1186/s13062-015-0081-6. PMC 4572633. PMID 26374501.
- Pliatsika V, Loher P, Telonis AG, Rigoutsos I (August 2016). "MINTbase: a framework for the interactive exploration of mitochondrial and nuclear tRNA fragments". Bioinformatics. 32 (16): 2481–9. doi:10.1093/bioinformatics/btw194. PMC 4978933. PMID 27153631.
- Kumar P, Mudunuri SB, Anaya J, Dutta A (January 2015). "tRFdb: a database for transfer RNA fragments". Nucleic Acids Research. 43 (Database issue): D141-5. doi:10.1093/nar/gku1138. PMC 4383946. PMID 25392422.
- Vincent RM, Wright BW, Jaschke PR (April 2019). "Measuring Amber Initiator tRNA Orthogonality in a Genomically Recoded Organism". ACS Synthetic Biology. 8 (4): 675–685. doi:10.1021/acssynbio.9b00021. PMID 30856316.
- Govindan A, Miryala S, Mondal S, Varshney U (November 2018). "Development of Assay Systems for Amber Codon Decoding at the Steps of Initiation and Elongation in Mycobacteria". Journal of Bacteriology. 200 (22). doi:10.1128/jb.00372-18. PMC 6199473. PMID 30181124.
- White RJ (March 1997). "Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control?". Trends in Biochemical Sciences. 22 (3): 77–80. doi:10.1016/S0968-0004(96)10067-0. PMID 9066256.
- Sharp S, Dingermann T, Söll D (September 1982). "The minimum intragenic sequences required for promotion of eukaryotic tRNA gene transcription". Nucleic Acids Research. 10 (18): 5393–406. doi:10.1093/nar/10.18.5393. PMC 320884. PMID 6924209.
- Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A (December 2007). "The expanding RNA polymerase III transcriptome". Trends in Genetics. 23 (12): 614–22. doi:10.1016/j.tig.2007.09.001. PMID 17977614.
- Tocchini-Valentini GD, Fruscoloni P, Tocchini-Valentini GP (December 2009). "Processing of multiple-intron-containing pretRNA". Proceedings of the National Academy of Sciences of the United States of America. 106 (48): 20246–51. Bibcode:2009PNAS..10620246T. doi:10.1073/pnas.0911658106. PMC 2787110. PMID 19910528.
- Abelson J, Trotta CR, Li H (May 1998). "tRNA splicing". The Journal of Biological Chemistry. 273 (21): 12685–8. doi:10.1074/jbc.273.21.12685. PMID 9582290.
- Soma A (2014). "Circularly permuted tRNA genes: their expression and implications for their physiological relevance and development". Frontiers in Genetics. 5: 63. doi:10.3389/fgene.2014.00063. PMC 3978253. PMID 24744771.
- Frank DN, Pace NR (1998). "Ribonuclease P: unity and diversity in a tRNA processing ribozyme". Annual Review of Biochemistry. 67 (1): 153–80. doi:10.1146/annurev.biochem.67.1.153. PMID 9759486.
- Ceballos M, Vioque A (2007). "tRNase Z". Protein and Peptide Letters. 14 (2): 137–45. doi:10.2174/092986607779816050. PMID 17305600.
- Randau L, Schröder I, Söll D (May 2008). "Life without RNase P". Nature. 453 (7191): 120–3. Bibcode:2008Natur.453..120R. doi:10.1038/nature06833. PMID 18451863.
- Weiner AM (October 2004). "tRNA maturation: RNA polymerization without a nucleic acid template". Current Biology. 14 (20): R883-5. doi:10.1016/j.cub.2004.09.069. PMID 15498478.
- Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Görlich D (February 1998). "Identification of a tRNA-specific nuclear export receptor". Molecular Cell. 1 (3): 359–69. doi:10.1016/S1097-2765(00)80036-2. PMID 9660920.
- Arts GJ, Fornerod M, Mattaj IW (March 1998). "Identification of a nuclear export receptor for tRNA". Current Biology. 8 (6): 305–14. doi:10.1016/S0960-9822(98)70130-7. PMID 9512417.
- Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW (December 1998). "The role of exportin-t in selective nuclear export of mature tRNAs". The EMBO Journal. 17 (24): 7430–41. doi:10.1093/emboj/17.24.7430. PMC 1171087. PMID 9857198.
- Yoshihisa T, Yunoki-Esaki K, Ohshima C, Tanaka N, Endo T (August 2003). "Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria". Molecular Biology of the Cell. 14 (8): 3266–79. doi:10.1091/mbc.E02-11-0757. PMC 181566. PMID 12925762.
- http://www.jbc.org/content/280/40/e37
- Clark BF (October 2006). "The crystal structure of tRNA" (PDF). Journal of Biosciences. 31 (4): 453–7. doi:10.1007/BF02705184. PMID 17206065.
- Holley RW, Apgar J, Everett GA, Madison JT, Marquisee M, Merrill SH, Penswick JR, Zamir A (March 1965). "Structure of a Ribonucleic Acid". Science. 147 (3664): 1462–5. Bibcode:1965Sci...147.1462H. doi:10.1126/science.147.3664.1462. PMID 14263761.
- "Obituary". The New York Times. July 4, 1991.
- "The Nobel Prize in Physiology or Medicine 1968". Nobel Foundation. Retrieved 2007-07-28.
- Ladner JE, Jack A, Robertus JD, Brown RS, Rhodes D, Clark BF, Klug A (November 1975). "Structure of yeast phenylalanine transfer RNA at 2.5 A resolution". Proceedings of the National Academy of Sciences of the United States of America. 72 (11): 4414–8. Bibcode:1975PNAS...72.4414L. doi:10.1073/pnas.72.11.4414. PMC 388732. PMID 1105583.
- Kim SH, Quigley GJ, Suddath FL, McPherson A, Sneden D, Kim JJ, Weinzierl J, Rich A (January 1973). "Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain". Science. 179 (4070): 285–8. Bibcode:1973Sci...179..285K. doi:10.1126/science.179.4070.285. PMID 4566654.
External links
| Wikimedia Commons has media related to TRNA. |