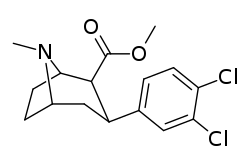
Dichloropane
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively.[1] In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H17Cl2NO2 |
| Molar mass | 314.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methylecgonidine is the direct precursor to this compound.[4]
Trans -CO2Me group
The thermodynamic isomer with a trans -CO2Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials.
- Tesofensine
- Brasofensine
- NS-2359 (GSK-372,475)
gollark: (It runs on LEU 235 Ox as a pulse reactor)
gollark: We even have an automatic power plant control system. If someone ctrl-t's the program to edit the code without flipping the manual control to off it could even melt!
gollark: Compromise: the power plant itself is TMI. The island is sheep island or whatever.
gollark: Ten Metre Island is online though not actually sending power anywhere.
gollark: * most
References
- Carroll FI, Blough BE, Nie Z, Kuhar MJ, Howell LL, Navarro HA (April 2005). "Synthesis and monoamine transporter binding properties of 3beta-(3',4'-disubstituted phenyl)tropane-2beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry. 48 (8): 2767–71. doi:10.1021/jm040185a. PMID 15828814.
- Ranaldi R, Anderson KG, Carroll FI, Woolverton WL (December 2000). "Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine". Psychopharmacology. 153 (1): 103–10. doi:10.1007/s002130000602. PMID 11255920.
- Cook CD, Carroll IF, Beardsley PM (December 2001). "Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat". Psychopharmacology. 159 (1): 58–63. doi:10.1007/s002130100891. PMID 11797070.
- Carroll FI, Mascarella SW, Kuzemko MA, Gao Y, Abraham P, Lewin AH, et al. (September 1994). "Synthesis, ligand binding, and QSAR (CoMFA and classical) study of 3 beta-(3'-substituted phenyl)-, 3 beta-(4'-substituted phenyl)-, and 3 beta-(3',4'-disubstituted phenyl)tropane-2 beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry. 37 (18): 2865–73. doi:10.1021/jm00044a007. PMID 8071935.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.