Escitalopram
Escitalopram, sold under the brand names Cipralex and Lexapro, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[2] Escitalopram is mainly used to treat major depressive disorder or generalized anxiety disorder.[2] It is taken by mouth.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛsəˈtæləˌpræm/ |
| Trade names | Cipralex, Lexapro, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603005 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Selective serotonin reuptake inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | ~56% |
| Metabolism | Liver, specifically the enzymes CYP3A4 and CYP2C19 |
| Elimination half-life | 27–32 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.244.188 |
| Chemical and physical data | |
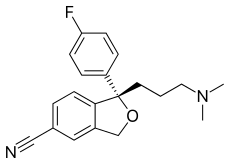
| Formula | C20H21FN2O |
| Molar mass | 324.392 g/mol (414.43 as oxalate) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include trouble sleeping, nausea, sexual problems, and feeling tired.[2] More serious side effects may include suicide in people under the age of 25.[2] It is unclear if use during pregnancy or breastfeeding is safe.[3] Escitalopram is the (S)-stereoisomer (left-handed version) of citalopram (which exists as a racemate), hence the name escitalopram.[2]
Escitalopram was approved for medical use in the United States in 2002.[2] Escitalopram is sometimes replaced by twice the dose of citalopram.[4] In 2017, it was the 20th most commonly prescribed medication in the United States with more than 25 million prescriptions.[5][6]
Medical uses
Escitalopram has FDA approval for the treatment of major depressive disorder in adolescents and adults, and generalized anxiety disorder in adults.[2] In European countries and the United Kingdom, it is approved for depression (MDD) and anxiety disorders, these include: general anxiety disorder (GAD), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and panic disorder with or without agoraphobia. In Australia it is approved for major depressive disorder.[7][8][9]
Depression
Escitalopram was approved by regulatory authorities for the treatment of major depressive disorder on the basis of four placebo controlled, double-blind trials, three of which demonstrated a statistical superiority over placebo.[10]
Controversy existed regarding the effectiveness of escitalopram compared with its predecessor, citalopram. The importance of this issue followed from the greater cost of escitalopram relative to the generic mixture of isomers of citalopram, prior to the expiration of the escitalopram patent in 2012, which led to charges of evergreening. Accordingly, this issue has been examined in at least 10 different systematic reviews and meta analyses. As of 2012, reviews had concluded (with caveats in some cases) that escitalopram is modestly superior to citalopram in efficacy and tolerability.[11][12][13][14]
A 2011 review concluded that second-generation antidepressants appear equally effective, although they may differ in onset and side effects.[15] Treatment guidelines issued by the National Institute of Health and Clinical Excellence and by the American Psychiatric Association generally reflect this viewpoint.[16][17]
In 2018, a systematic review and network meta-analysis comparing the efficacy and acceptability of 21 antidepressant drugs showed escitalopram to be one of the most effective.[18]
Anxiety disorder
Escitalopram appears to be effective in treating general anxiety disorder, with relapse on escitalopram at 20% rather than placebo at 50%.[19]
Escitalopram appears effective in treating social anxiety disorder.[20]
Other
Escitalopram is effective in reducing the symptoms of premenstrual syndrome, whether taken continuously or in the luteal phase only.[21] There are no good data available for escitalopram as treatment for seasonal affective disorder as of 2011.[22] SSRIs do not appear to be useful for preventing tension headaches or migraines.[23][24]
Side effects
Escitalopram, like other SSRIs, has been shown to affect sexual functions causing side effects such as decreased libido, delayed ejaculation, and anorgasmia.[25][26]
An analysis conducted by the FDA found a statistically insignificant 1.5 to 2.4-fold (depending on the statistical technique used) increase of suicidality among the adults treated with escitalopram for psychiatric indications.[27][28][29] The authors of a related study note the general problem with statistical approaches: due to the rarity of suicidal events in clinical trials, it is hard to draw firm conclusions with a sample smaller than two million patients.[30]
Escitalopram is not associated with weight gain. For example, 0.6 kg mean weight change after 6 months of treatment with escitalopram for depression was insignificant and similar to that with placebo (0.2 kg).[31]
Citalopram and escitalopram are associated with dose-dependent QT interval prolongation[32] and should not be used in those with congenital long QT syndrome or known pre-existing QT interval prolongation, or in combination with other medicines that prolong the QT interval. ECG measurements should be considered for patients with cardiac disease, and electrolyte disturbances should be corrected before starting treatment. In December 2011, the UK implemented new restrictions on the maximum daily doses at 20 mg for adults and 10 mg for those older than 65 years or with liver impairment.[33][34] There are concerns of higher rates of QT prolongation and torsades de pointes compared with other SSRIs.[35][36] The U.S. Food and Drug Administration and Health Canada did not similarly order restrictions on escitalopram dosage, only on its predecessor citalopram.[37]
Very common effects
Very common effects (>10% incidence) include:[38][39][40][41]
- Headache (24%)
- Nausea (18%)
- Ejaculation disorder (9–14%)
- Somnolence (4–13%)
- Insomnia (7–12%)
Common (1–10% incidence)
Common effects (1–10% incidence) include:
- Insomnia
- Somnolence (sleepiness)
- Dizziness
- Paraesthesia
- Tremor
- Decreased or increased appetite
- Anxiety
- Restlessness
- Abnormal dreams
- Libido decreased
- Anorgasmia
- Sinusitis (nasal congestion)
- Yawning
- Diarrhea
- Constipation
- Vomiting
- Dry mouth
- Excessive sweating
- Arthralgia (joint pain)
- Myalgia (muscular aches and pains)
- Fatigue
- Pyrexia (fever)
- Impotence (erectile dysfunction)
Psychomotor effects
The most common effect is fatigue or somnolence, particularly in older adults,[42] although patients with pre-existing daytime sleepiness and fatigue may experience paradoxical improvement of these symptoms.[43] Escitalopram has not been shown to affect serial reaction time, logical reasoning, serial subtraction, multitask, or MacWorth clock task performance.[44]
Discontinuation symptoms
Escitalopram discontinuation, particularly abruptly, may cause certain withdrawal symptoms such as "electric shock" sensations[45] (also known as "brain shivers" or "brain zaps"), dizziness, acute depressions and irritability, as well as heightened senses of akathisia.[46]
Sexual dysfunction
Some people experience persistent sexual side effects after they stop taking SSRIs.[47] This is known as post-SSRI sexual dysfunction (PSSD). Common symptoms include genital anesthesia, erectile dysfunction, anhedonia, decreased libido, premature ejaculation, vaginal lubrication issues, and nipple insensitivity in women. Rates are unknown, and there is no established treatment.[48] Lenze et al. in their randomized, placebo comparative study found that sexual adverse effects were not significant (p value of 7 percent).[42]
Pregnancy
Antidepressant exposure (including escitalopram) is associated with shorter duration of pregnancy (by three days), increased risk of preterm delivery (by 55%), lower birth weight (by 75 g), and lower Apgar scores (by <0.4 points). Antidepressant exposure is not associated with an increased risk of spontaneous abortion.[49] There is a tentative association of SSRI use during pregnancy with heart problems in the baby.[50] The advantages of their use during pregnancy may thus outweigh the possible negative effects on the baby.[50]
Overdose
Excessive doses of escitalopram usually cause relatively minor untoward effects, such as agitation and tachycardia. However, dyskinesia, hypertonia, and clonus may occur in some cases. Therapeutic blood levels of escitalopram are usually in the range of 20–80 μg/L but may reach 80–200 μg/L in the elderly, patients with hepatic dysfunction, those who are poor CYP2C19 metabolizers or following acute overdose. Monitoring of the drug in plasma or serum is generally accomplished using chromatographic methods. Chiral techniques are available to distinguish escitalopram from its racemate, citalopram.[51][52][53]
Pharmacology
Mechanism of action
| Site | Ki (nM) |
|---|---|
| SERT | 0.8-1.1 |
| NET | 7,800 |
| DAT | 27,400 |
| 5-HT1A | >1,000 |
| 5-HT2A | >1,000 |
| 5-HT2C | 2,500 |
| α1 | 3,900 |
| α2 | >1,000 |
| D2 | >1,000 |
| H1 | 2,000 |
| mACh | 1,240 |
Escitalopram increases intrasynaptic levels of the neurotransmitter serotonin by blocking the reuptake of the neurotransmitter into the presynaptic neuron. Of the SSRIs currently available, escitalopram has the highest selectivity for the serotonin transporter (SERT) compared to the norepinephrine transporter (NET), making the side-effect profile relatively mild in comparison to less-selective SSRIs.[55]
Escitalopram is a substrate of P-glycoprotein and hence P-glycoprotein inhibitors such as verapamil and quinidine may improve its blood brain barrier penetrability.[56] In a preclinical study in rats combining escitalopram with a P-glycoprotein inhibitor, its antidepressant-like effects were enhanced.[56]
Interactions
Escitalopram, similarly to other SSRIs, inhibits CYP2D6 and hence may increase plasma levels of a number of CYP2D6 substrates such as aripiprazole, risperidone, tramadol, codeine, etc. As escitalopram is only a weak inhibitor of CYP2D6, analgesia from tramadol may not be affected.[57] Escitalopram should be taken with caution when using St. John's wort.[58] Exposure to escitalopram is increased moderately, by about 50%, when it is taken with omeprazole. The authors of this study suggested that this increase is unlikely to be of clinical concern.[59] Caution should be used when taking cough medicine containing dextromethorphan (DXM) as serotonin syndrome has been reported.[60]
Bupropion has been found to significantly increase citalopram plasma concentration and systemic exposure; as of April 2018 the interaction with escitalopram had not been studied, but some monographs warned of the potential interaction.[61]
Escitalopram can also prolong the QT interval and hence it is not recommended in patients that are concurrently on other medications that also have the ability to prolong the QT interval. These drugs include antiarrhythmics, antipsychotics, tricyclic antidepressants, some antihistamines (astemizole, mizolastine) and some antiretrovirals (ritonavir, saquinavir, lopinavir).[33] As an SSRI, escitalopram should generally not be given concurrently with MAOIs.[55]
Chemistry
Escitalopram is the (S)-stereoisomer (left-handed version) of the racemate citalopram, which is responsible for its name: escitalopram.[2] The (R)-stereoisomer (R-citalopram, the right-handed version) is not thought to have useful effects for treating depression.[62]
History
Escitalopram was developed in close cooperation between Lundbeck and Forest Laboratories. Its development was initiated in the summer of 1997, and the resulting new drug application was submitted to the U.S. FDA in March 2001. The short time (3.5 years) it took to develop escitalopram can be attributed to the previous extensive experience of Lundbeck and Forest with citalopram, which has similar pharmacology.[63]
The FDA issued the approval of escitalopram for major depression in August 2002 and for generalized anxiety disorder in December 2003. On May 23, 2006, the FDA approved a generic version of escitalopram by Teva.[64] On July 14 of that year, however, the U.S. District Court of Delaware decided in favor of Lundbeck regarding the patent infringement dispute and ruled the patent on escitalopram valid.[65]
In 2006, Forest Laboratories was granted an 828-day (2 years and 3 months) extension on its US patent for escitalopram.[66] This pushed the patent expiration date from December 7, 2009 to September 14, 2011. Together with the 6-month pediatric exclusivity, the final expiration date was March 14, 2012.
Society and culture
Allegations of illegal marketing
In 2004, separate civil suits alleging illegal marketing of citalopram and escitalopram for use by children and teenagers by Forest were initiated by two whistleblowers: a physician named Joseph Piacentile and a Forest salesman named Christopher Gobble.[67] In February 2009, the suits were joined. Eleven states and the District of Columbia filed notices of intent to intervene as plaintiffs in the action.
The suits alleged that Forest illegally engaged in off-label promotion of Lexapro for use in children; hid the results of a study showing lack of effectiveness in children; paid kickbacks to physicians to induce them to prescribe Lexapro to children; and conducted so-called "seeding studies" that were, in reality, marketing efforts to promote the drug's use by doctors.[68][69] Forest denied the allegations[70] but ultimately agreed to settle with the plaintiffs for over $313 million.[71]
Brand names
Escitalopram is sold under many brand names worldwide such as Cipralex and Lexapro.[1]
References
- drugs.com Drugs.com international: Escitalopram Page accessed April 25, 2015
- "X". The American Society of Health-System Pharmacists. Retrieved 28 December 2017.
- "Escitalopram (Lexapro) Use During Pregnancy". Drugs.com. Retrieved 31 December 2017.
- "Protocol for switching patients from escitalopram to citalopram". NHS. 2015. Retrieved 13 February 2018.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Escitalopram Oxalate Drug Usage Statistics". ClinCalc. 23 December 2019. Retrieved 11 April 2020.
- "Escitalopram oxalate". Australian Prescriber. 26: 146–151. 2003. doi:10.18773/austprescr.2003.107.
- "Lundbeck's Cipralex gets EU ok for OCD treatment". Reuters. 2007-01-12. Retrieved 2020-03-15.
- "Cipralex 10 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Retrieved 2020-03-15.
- "www.accessdata.fda.gov" (PDF).
- Ramsberg J, Asseburg C, Henriksson M (2012). "Effectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness model". PLOS ONE. 7 (8): e42003. Bibcode:2012PLoSO...742003R. doi:10.1371/journal.pone.0042003. PMC 3410906. PMID 22876296.
- Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, Churchill R, Watanabe N, Barbui C (July 2012). "Citalopram versus other anti-depressive agents for depression". The Cochrane Database of Systematic Reviews. 7 (7): CD006534. doi:10.1002/14651858.CD006534.pub2. PMC 4204633. PMID 22786497.
- Favré P (February 2012). "[Clinical efficacy and achievement of a complete remission in depression: increasing interest in treatment with escitalopram]" [Clinical efficacy and achievement of a complete remission in depression: Increasing interest in treatment with escitalopram]. L'Encephale (in French). 38 (1): 86–96. doi:10.1016/j.encep.2011.11.003. PMID 22381728.
- Sicras-Mainar A, Navarro-Artieda R, Blanca-Tamayo M, Gimeno-de la Fuente V, Salvatella-Pasant J (December 2010). "Comparison of escitalopram vs. citalopram and venlafaxine in the treatment of major depression in Spain: clinical and economic consequences". Current Medical Research and Opinion. 26 (12): 2757–64. doi:10.1185/03007995.2010.529430. PMID 21034375. S2CID 43179425.
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN (December 2011). "Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis". Annals of Internal Medicine. 155 (11): 772–85. doi:10.7326/0003-4819-155-11-201112060-00009. PMID 22147715.
Current evidence does not warrant recommending a particular second-generation antidepressant on the basis of differences in efficacy. Differences in onset of action and adverse events may be considered when choosing a medication.
- "CG90 Depression in adults: full guidance".
- Practice Guideline for the Treatment of Patients With Major Depressive Disorder (PDF). APA Practice Guidelines (3rd ed.). 2010.
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR (April 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. 391 (10128): 1357–1366. doi:10.1016/S0140-6736(17)32802-7. PMC 5889788. PMID 29477251.
- Bech P, Lönn SL, Overø KF (February 2010). "Relapse prevention and residual symptoms: a closer analysis of placebo-controlled continuation studies with escitalopram in major depressive disorder, generalized anxiety disorder, social anxiety disorder, and obsessive-compulsive disorder". The Journal of Clinical Psychiatry. 71 (2): 121–9. doi:10.4088/JCP.08m04749blu. PMID 19961809.
- Baldwin DS, Asakura S, Koyama T, Hayano T, Hagino A, Reines E, Larsen K (June 2016). "Efficacy of escitalopram in the treatment of social anxiety disorder: A meta-analysis versus placebo". European Neuropsychopharmacology. 26 (6): 1062–9. doi:10.1016/j.euroneuro.2016.02.013. PMID 26971233.
- Marjoribanks J, Brown J, O'Brien PM, Wyatt K (June 2013). "Selective serotonin reuptake inhibitors for premenstrual syndrome". The Cochrane Database of Systematic Reviews (6): CD001396. doi:10.1002/14651858.CD001396.pub3. PMC 7073417. PMID 23744611.
- Thaler K, Delivuk M, Chapman A, Gaynes BN, Kaminski A, Gartlehner G (December 2011). "Second-generation antidepressants for seasonal affective disorder". The Cochrane Database of Systematic Reviews (12): CD008591. doi:10.1002/14651858.CD008591.pub2. PMID 22161433.
- Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L (May 2015). "Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults". The Cochrane Database of Systematic Reviews (5): CD011681. doi:10.1002/14651858.CD011681. PMC 6864942. PMID 25931277.
- Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L (April 2015). "Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults". The Cochrane Database of Systematic Reviews. 4: CD002919. doi:10.1002/14651858.CD002919.pub3. hdl:2434/273130. PMC 6513227. PMID 25829028.
- Clayton A, Keller A, McGarvey EL (March 2006). "Burden of phase-specific sexual dysfunction with SSRIs". Journal of Affective Disorders. 91 (1): 27–32. doi:10.1016/j.jad.2005.12.007. PMID 16430968.
- "Lexapro prescribing information" (PDF).
- Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". Retrieved 2007-05-13.
- Stone MB, Jones ML (2006-11-17). "Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults" (PDF). Overview for December 13 Meeting of Pharmacological Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Retrieved 2007-09-22.
- Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Pharmacological Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Retrieved 2007-09-22.
- Khan A, Schwartz K (2007). "Suicide risk and symptom reduction in patients assigned to placebo in duloxetine and escitalopram clinical trials: analysis of the FDA summary basis of approval reports". Annals of Clinical Psychiatry. 19 (1): 31–6. doi:10.1080/10401230601163550. PMID 17453659.
- Baldwin DS, Reines EH, Guiton C, Weiller E (October 2007). "Escitalopram therapy for major depression and anxiety disorders". The Annals of Pharmacotherapy. 41 (10): 1583–92. doi:10.1345/aph.1K089. PMID 17848424. S2CID 25063917.
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH (January 2013). "QT interval and antidepressant use: a cross sectional study of electronic health records". BMJ. 346: f288. doi:10.1136/bmj.f288. PMC 3558546. PMID 23360890.
- "Citalopram and escitalopram: QT interval prolongation—new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings". Medicines and Healthcare products Regulatory Agency. December 2011. Retrieved March 5, 2013.
- van Gorp F, Whyte IM, Isbister GK (September 2009). "Clinical and ECG effects of escitalopram overdose" (PDF). Annals of Emergency Medicine. 54 (3): 404–8. doi:10.1016/j.annemergmed.2009.04.016. PMID 19556032.
- "Towards better patient care: drugs to avoid in 2017". Retrieved 2018-09-17.
- Drugs To Avoid 2017 PDF Download
- Hasnain M, Howland RH, Vieweg WV (July 2013). "Escitalopram and QTc prolongation". Journal of Psychiatry & Neuroscience. 38 (4): E11. doi:10.1503/jpn.130055. PMC 3692726. PMID 23791140.
- "Lexapro (escitalopram) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 27 November 2013.
- "Cipralex 5, 10 and 20 mg film-coated tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. 2 October 2013. Retrieved 27 November 2013.
- "ESCITALOPRAM-LUPIN TABLETS (LUPIN AUSTRALIA PTY. LTD)" (PDF). TGA eBusiness Services. Lupin Australia Pty Ltd. 21 December 2011. Retrieved 27 November 2013.
- "ESCITALOPRAM (escitalopram oxalate) tablet, film coated [Apotex Corp.]". DailyMed. Apotex Corp. September 2013. Retrieved 27 November 2013.
- Lenze, Eric J. (2009-05-20). "Escitalopram Treatment of Generalized Anxiety Disorder in Older Adults—Reply". JAMA. 301 (19): 1987. doi:10.1001/jama.2009.652. ISSN 0098-7484.
- Shen, Jianhua; Hossain, Naheed; Streiner, David L.; Ravindran, Aruu V.; Wang, Xuehua; Deb, Prativa; Huang, Xin; Sun, Frank; Shapiro, Colin M. (November 2011). "Excessive daytime sleepiness and fatigue in depressed patients and therapeutic response of a sedating antidepressant". Journal of Affective Disorders. 134 (1–3): 421–426. doi:10.1016/j.jad.2011.04.047. ISSN 0165-0327. PMID 21616541.
- Rosekind MR, Gregory KB, Mallis MM (December 2006). "Alertness management in aviation operations: enhancing performance and sleep". Aviation, Space, and Environmental Medicine. 77 (12): 1256–65. doi:10.3357/asem.1879.2006. PMID 17183922.
- Prakash O, Dhar V (June 2008). "Emergence of electric shock-like sensations on escitalopram discontinuation". Journal of Clinical Psychopharmacology. 28 (3): 359–60. doi:10.1097/JCP.0b013e3181727534. PMID 18480703.
- "Lexapro (Escitalopram Oxalate) Drug Information: Warnings and Precautions - Prescribing Information at RxList". Archived from the original on 2008-06-16. Retrieved 2015-08-09.
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. pp. 449. ISBN 9780890425558.
- Bala A, Nguyen HM, Hellstrom WJ (January 2018). "Post-SSRI Sexual Dysfunction: A Literature Review". Sexual Medicine Reviews. 6 (1): 29–34. doi:10.1016/j.sxmr.2017.07.002. PMID 28778697.
- Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, Rehm J, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A (April 2013). "Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis". JAMA Psychiatry. 70 (4): 436–43. doi:10.1001/jamapsychiatry.2013.684. PMID 23446732.
- Gentile S (1 July 2015). "Early pregnancy exposure to selective serotonin reuptake inhibitors, risks of major structural malformations, and hypothesized teratogenic mechanisms". Expert Opinion on Drug Metabolism & Toxicology. 11 (10): 1585–97. doi:10.1517/17425255.2015.1063614. PMID 26135630. S2CID 43329515.
- van Gorp F, Whyte IM, Isbister GK (September 2009). "Clinical and ECG effects of escitalopram overdose". Annals of Emergency Medicine. 54 (3): 404–8. doi:10.1016/j.annemergmed.2009.04.016. PMID 19556032.
- Haupt D (October 1996). "Determination of citalopram enantiomers in human plasma by liquid chromatographic separation on a Chiral-AGP column". Journal of Chromatography B. 685 (2): 299–305. doi:10.1016/s0378-4347(96)00177-6. PMID 8953171.
- Baselt RC (2008). Disposition of toxic drugs and chemicals in man (8th ed.). Foster City, Ca: Biomedical Publications. pp. 552–553. ISBN 978-0962652370.
- Sanchez C, Reines EH, Montgomery SA (July 2014). "A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike?". International Clinical Psychopharmacology. 29 (4): 185–96. doi:10.1097/YIC.0000000000000023. PMC 4047306. PMID 24424469.
- Brunton L, Chabner B, Knollman B. Goodman and Gilman's The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.
- O'Brien FE, O'Connor RM, Clarke G, Dinan TG, Griffin BT, Cryan JF (October 2013). "P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents". Neuropsychopharmacology. 38 (11): 2209–19. doi:10.1038/npp.2013.120. PMC 3773671. PMID 23670590.
- Noehr-Jensen L, Zwisler ST, Larsen F, Sindrup SH, Damkier P, Brosen K (December 2009). "Escitalopram is a weak inhibitor of the CYP2D6-catalyzed O-demethylation of (+)-tramadol but does not reduce the hypoalgesic effect in experimental pain". Clinical Pharmacology and Therapeutics. 86 (6): 626–33. doi:10.1038/clpt.2009.154. PMID 19710642.
- Karch, Amy (2006). 2006 Lippincott's Nursing Drug Guide. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo: Lippincott Williams & Wilkins. ISBN 978-1-58255-436-5.
- Malling D, Poulsen MN, Søgaard B (September 2005). "The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects". British Journal of Clinical Pharmacology. 60 (3): 287–90. doi:10.1111/j.1365-2125.2005.02423.x. PMC 1884771. PMID 16120067.
- Arcega, V.; Ghali, W.; Wolfe, W. (August 7, 2017), "Serotonin syndrome caused by drug to drug interaction between escitalopram and dextromethorphan", BMJ Case Reports
- "Drug interactions between bupropion and Lexapro". Drugs.com. Retrieved 22 April 2018.
- "Citalopram and escitalopram". Meyler's Side Effects of Drugs (6 ed.). Elsevier. 2016. p. 383.
- "2000 Annual Report. p 28 and 33" (PDF). Lundbeck. 2000. Retrieved 2007-04-07.
- Hitti M. "FDA OKs Generic Depression Drug – Generic Version of Lexapro Gets Green Light". WebMD. Retrieved 2007-10-10.
- Laforte M (2006-07-14). "US court upholds Lexapro patent". FirstWord. Retrieved 2007-10-10.
- "Forest Laboratories Receives Patent Term Extension for Lexapro" (Press release). PRNewswire-FirstCall. 2006-03-02. Retrieved 2009-01-19.
- "Forest Laboratories: A Tale of Two Whistleblowers" article by Alison Frankel in The American Lawyer February 27, 2009
- United States of America v. Forest Laboratories Full text of the federal complaint filed in the US District Court for the district of Massachusetts
- "Drug Maker Is Accused of Fraud" article by Barry Meier and Benedict Carey in The New York Times February 25, 2009
- "Forest Laboratories, Inc. Provides Statement in Response to Complaint Filed by U.S. Government" Forest press-release. February 26, 2009.
- "Drug Maker Forest Pleads Guilty; To Pay More Than $313 Million to Resolve Criminal Charges and False Claims Act Allegations". www.justice.gov. 2010-09-15.
Cited texts
- Royal Pharmaceutical Society of Great Britain (September 2009). British National Formulary (BNF 58). UK: BMJ Group and RPS Publishing. ISBN 978-0-85369-778-7.
Further reading
- Harris G (1 September 2009). "A Peek at How Forest Laboratories Pushed Lexapro". The New York Times. Retrieved 11 April 2020.