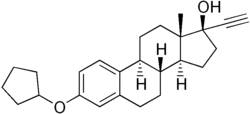
Quinestrol
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer.[2][3] It is taken once per week to once per month by mouth.[4][5][6][7]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Estrovis, others |
| Other names | Quinoestrol; Quinestrenol; Quinoestrenol; Ethinylestradiol 3-cyclopentyl ether; EECPE; EE2CPE; W-3566; 3-(Cyclopentyloxy)-17α-ethynylestra-1,3,5(10)-trien-17β-ol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| Drug class | Estrogen; Estrogen ether |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | >120 hours (>5 days)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.277 |
| Chemical and physical data | |
| Formula | C25H32O2 |
| Molar mass | 364.529 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
Quinestrol has been used as the estrogen component in menopausal hormone therapy and in combined hormonal birth control.[2][3] It has also occasionally been used in the treatment of breast cancer and prostate cancer, as well as to suppress lactation.[2][3][8] On its own as an estrogen, quinestrol was taken once per week by mouth.[4] As a combined birth control pill, it was used together with quingestanol acetate and was taken once per month by mouth.[5][6][7]
Pharmacology
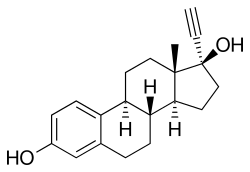
Quinestrol is a prodrug of ethinylestradiol (EE), with no estrogenic activity of its own.[3][9][10] It is taken orally and has prolonged activity following a single dose,[9][10] with a very long biological half-life of more than 120 hours (5 days) due to enhanced lipophilicity and storage in fat.[3][1] Because of its much longer half-life, quinestrol is two to three times as potent as EE.[3] Also because of its long half-life, quinestrol can be taken once a week or once a month.[3][4][5][6][7]
Following administration, quinestrol is absorbed via the lymphatic system, is stored in adipose tissue, and is gradually released from adipose tissue.[11]
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) expression assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
| Estrogen | Type | Class | ETD (2–3 weeks) | EPD (2–3 weeks) | MSD (2–3 weeks) | MSD (daily) | OID (daily) | TSD (daily) |
|---|---|---|---|---|---|---|---|---|
| Estradiol (non-micronized) | Bioidentical | Steroidal | 30 mg | ≥120–300 mg | 120 mg | 6 mg | ? | ? |
| Estradiol (micronized) | Bioidentical | Steroidal | 6–12 mg | 60–80 mg | 14–42 mg | 1–2 mg | >5 mg | >8 mg |
| Estradiol valerate | Bioidentical | Steroidal | 6–12 mg | 60–80 mg | 14–42 mg | 1–2 mg | ? | >8 mg |
| Estradiol benzoate | Bioidentical | Steroidal | ? | 60–140 mg | ? | ? | ? | ? |
| Estriol | Bioidentical | Steroidal | 20 mga | 120–150 mgb | 28–126 mg | 1–6 mg | >5 mg | ? |
| Estriol succinate | Bioidentical | Steroidal | ? | 140–150 mgb | 28–126 mg | 2–6 mg | ? | ? |
| Estrone sulfate | Bioidentical | Steroidal | 12 mg | 60 mg | 42 mg | 2 mg | ? | ? |
| Conjugated estrogens | Natural | Steroidal | 5–12 mg | 60–80 mg | 8.4–25 mg | 0.625–1.25 mg | >3.75 mg | 7.5 mg |
| Ethinylestradiol | Synthetic | Steroidal | 200 μg | 1–2 mg | 280 μg | 20–40 μg | 100 μg | 100 μg |
| Mestranol | Synthetic | Steroidal | 300 μg | 1.5–3.0 mg | 300–600 μg | 25–30 μg | >80 μg | ? |
| Quinestrol | Synthetic | Steroidal | 300 μg | 2–4 mg | 500 μg | 25–50 μg | ? | ? |
| Methylestradiol | Synthetic | Steroidal | ? | 2 mg | ? | ? | ? | ? |
| Diethylstilbestrol | Synthetic | Nonsteroidal | 2.5 mg | 20–30 mg | 11 mg | 0.5–2.0 mg | >5 mg | 3 mg |
| Diethylstilbestrol dipropionate | Synthetic | Nonsteroidal | ? | 15–30 mg | ? | ? | ? | ? |
| Dienestrol | Synthetic | Nonsteroidal | 5 mg | 30–40 mg | 42 mg | 0.5–4.0 mg | ? | ? |
| Dienestrol diacetate | Synthetic | Nonsteroidal | 3–5 mg | 30–60 mg | ? | ? | ? | ? |
| Hexestrol | Synthetic | Nonsteroidal | ? | 70–110 mg | ? | ? | ? | ? |
| Chlorotrianisene | Synthetic | Nonsteroidal | ? | >100 mg | ? | ? | >48 mg | ? |
| Methallenestril | Synthetic | Nonsteroidal | ? | 400 mg | ? | ? | ? | ? |
| Footnotes: a = Very variable, often higher. b = In divided doses, 3x/day; irregular and atypical proliferation. Sources: See template. | ||||||||
Chemistry
Quinestrol, also known as ethinylestradiol 3-cyclopentyl ether (EE2CPE), is a synthetic estrane steroid and a derivative of estradiol.[12][13] It is an estrogen ether, specifically the C3 cyclopentyl ether of ethinylestradiol (17α-ethynylestradiol).[12][13] Closely related estrogens include mestranol (ethinylestradiol 3-methyl ether) and ethinylestradiol sulfonate (EES; Turisteron; ethinylestradiol 3-isopropylsulfonate).[12][13]
History
Quinestrol was developed and introduced for medical use in the 1960s.[14]
Society and culture
Generic names
Quinestrol is the generic name of the drug and its INN, USAN, and BAN.[12][13][15][16] It is also known by its former developmental code name W-3566.[12][13][15][16]
Brand names
Quinestrol has been marketed under brand names including Agalacto-Quilea, Basaquines, Eston, Estrovis, Estrovister, Plestrovis, Qui-Lea, Soluna, and Yueketing, among others.[12][13][15][16]
Availability
Quinestrol was marketed as Estrovis in the United States by Parke-Davis and as Qui-Lea in Argentina,[13] but is reportedly not currently marketed.[3] However, it does appear to still be available as an oral contraceptive in combination with progestins in Argentina and China.[16]
One tablet form available in China consists of 6 mg levonorgestrel and 3 mg quinestrol; it is used as a prescription "long-term" oral contraceptive, with one dose taken each month.[16][17] It is sold under various brand names including Yuèkětíng (Chinese: 悦可婷) and Àiyuè (Chinese: 艾悦). A version with the racemic norgestrel in place of levonorgestrel also appears to be available.[16]
Veterinary use
Rodents
The Chinese levonorgestrel/quinestrol 2:1 formula is known as EP-1 in veterinary practice. It is known to have some organ-specific effects on the Mongolian gerbil as measured by receptor mRNA expression.[18] Incorporated into baits at a concentration of 50 ppm, EP-1 has been used to control wild Mongolian gerbil populations with some success.[19]
References
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 248–. ISBN 978-3-642-60107-1.
- Christoph Zink (1 January 1988). Dictionary of Obstetrics and Gynecology. Walter de Gruyter. pp. 204–. ISBN 978-3-11-085727-6.
- A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 381–. ISBN 978-1-59259-700-0.
- H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 60–. ISBN 978-1-4612-5525-3.
- J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 85, 358, 367. ISBN 978-94-009-8195-9.
- D F Hawkins; M G Elder (22 October 2013). Human Fertility Control: Theory and Practice. Elsevier Science. pp. 92–94. ISBN 978-1-4831-6361-1.
- Chemical Contraception. Macmillan International Higher Education. 18 June 1974. pp. 61–. ISBN 978-1-349-02287-8.
- Helmuth Vorherr (2 December 2012). The Breast: Morphology, Physiology, and Lactation. Elsevier Science. pp. 201–203. ISBN 978-0-323-15726-1.
- Epstein JA (1967). "Prolonged menstrual response of patients with gonadal failure following quinestrol administration". Int. J. Fertil. 12 (2): 181–6. PMID 6033895.
- Giannina T, Meli A (April 1969). "Prolonged oestrogenic activity in rats after single oral administration of ethinyloestradiol-3-cyclopentyl ether". J. Pharm. Pharmacol. 21 (4): 271–2. doi:10.1111/j.2042-7158.1969.tb08247.x. PMID 4390151.
- Wallach, Edward E.; Hammond, Charles B.; Maxson, Wayne S. (1982). "Current status of estrogen therapy for the menopause". Fertility and Sterility. 37 (1): 5–25. doi:10.1016/S0015-0282(16)45970-4. ISSN 0015-0282.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 522–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 905–. ISBN 978-3-88763-075-1.
- Medical Gynaecology and Sociology. Medical and Scientific Services Limited. 1967.
[...] J. Fertil., 1967, 12, 2) contains 23 papers presented at a symposium on QUINESTROL. Quinestrol is a newly-developed synthetic steroid, and is the cyclo-pentyl ether of a ethinyl oestradiol.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 243–. ISBN 978-94-011-4439-1.
- https://www.drugs.com/international/quinestrol.html
- "悦可婷 左炔诺孕酮炔雌醚片 6片/盒". Tmall. Archived from the original on 2018-07-16. Retrieved 2018-07-16.
初次服药时,于月经来潮的当天算起第五天午饭后服药一次,间隔20天服第二次,以后就以第二次服药日为每月的服药日期,每月服一片。原服用短效口服避孕药改服长效避孕药时,可在服完22片后的第二天接服长效避孕药一片,以后每月按开始服长效避孕药的同一日期服药一片。
- Lv, Xiaohui; Shi, Dazhao (January 2012). "Combined Effects of Levonorgestrel and Quinestrol on Reproductive Hormone Levels and Receptor Expression in Females of the Mongolian Gerbil". Zoological Science. 29 (1): 37–42. doi:10.2108/zsj.29.37. PMID 22233494.
- FU, Heping; ZHANG, Jinwei; SHI, Dazhao; WU, Xiaodong (September 2013). "Effects of levonorgestrel-quinestrol (EP-1) treatment on Mongolian gerbil wild populations: a case study". Integrative Zoology. 8 (3): 277–284. doi:10.1111/1749-4877.12018. PMID 24020466.