Fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds; most commonly those that occur in living beings or in food.[1]
| Types of fats in food |
|---|
| See also |
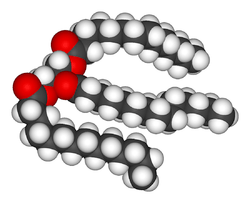
The term often refers specifically to triglycerides (triple esters of glycerol), that are the main components of vegetable oils and of fatty tissue in animals and humans;[2] or, even more narrowly, to triglycerides that are solid or semisolid at room temperature, thus excluding oils. The term may also be used more broadly as a synonym of lipid -- any substance of biological relevance, composed of carbon, hydrogen, or oxygen, that is insoluble in water but soluble in non-polar solvents.[1] In this sense, besides the triglycerides, the term would include several other types of compounds like phospholipids (such as lecithin), sterols (such as cholesterol), and waxes (such as beeswax),[1] which are usually present in human diet in smaller amounts.[2]
Fats are one of the three main macronutrient groups in human diet, along with carbohydrates and proteins,[1][3] and the main components of common food products like milk, butter, tallow, lard, bacon, and cooking oils. They are a major and dense source of food energy for many animals and play important structural and metabolic functions, in most living beings, including energy storage, waterproofing, and thermal insulation.[4] The human body can produce the fat that it needs from other food ingredients, except for a few essential fatty acids that must be included in the diet. Dietary fats are also the carriers of some flavor and aroma ingredients and vitamins that are not water-soluble.[2]
Chemical structure
The most important elements in the chemical makeup of fats are the fatty acids. The molecule of a fatty acid consists of a carboxyl group HO(O=)C– connected to an unbranched alkyl group –(CH
x)
nH: namely, a chain of carbon atoms, joined by single, double, or (more rarely) triple bonds, with all remaining free bonds filled by hydrogen atoms[5]
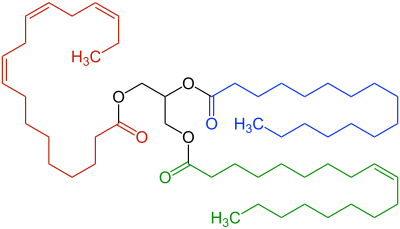
The most common type of fat, in human diet and most living beings, is a triglyceride, an ester of the triple alcohol glycerol H(–CHOH–)
3H and three fatty acids. The molecule of a trigliceride can be described as resulting from a condensation reaction (specifically, esterification) between each of glycerol's –OH groups and the HO– part of the carboxyl group HO(O=)C– of each fatty acid, forming an ester bridge –O–(O=)C– with elimination of a water molecule H
2O.
Other less common types of fats include diglycerides and monoglycerides, where the esterification is limited to two or just one of glycerol's –OH groups. Other alcohols, such as cetyl alcohol (predominant in spermaceti), may replace glycerol. In the phospholipids, one of the fatty acids is replaced by phosphoric acid or a monoester thereof.
Conformation
The shape of fat and fatty acid molecules is usually not well-defined. Any two parts of a molecule that are connected by just one single bond are free to rotate about that bond. Thus a fatty acid molecule with n simple bonds can be deformed in n-1 independent ways (counting also rotation of the terminal methyl group).
Such rotation cannot happen across a double bond, except by breaking and then reforming it with one of the halves of the molecule rotated by 180 degrees, which requires crossing a significant energy barrier. Thus a fat or fatty acid molecule with double bonds (excluding at the very end of the chain) can have multiple cis-trans isomers with significantly different chemical and biological properties. Each double bond reduces the number of conformational degrees of freedom by one. Each triple bond forces the four nearest carbons to lie in a straight line, removing two degrees of freedom.
It follows that depictions of "saturated" fatty acids with no double bonds (like stearic) having a "straight zig-zag" shape, and those with one cis bond (like oleic) being bent in an "elbow" shape are somewhat misleading. While the latter are a little less flexible, both can be twisted to assume similar straight or elbow shapes. In fact, outside of some specific contxts like crystals or bilayer membranes, both are more likely to be found in randomly contorted configurations than in either of those two shapes.
Examples
| Stearic acid saturated |
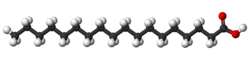 |
|---|---|
| Oleic acid unsaturated cis-8) |
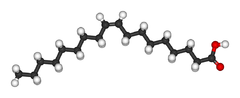 |
| Elaidic acid unsaturated trans-8) |
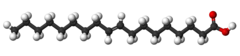 |
| Vaccenic acid unsaturated trans-11) |
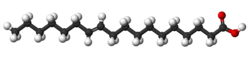 |
Stearic acid is a saturated fatty acid (with only single bonds) found in animal fats, and is the intended product in full hydrogenation.
Oleic acid has a double bond (thus being "unsaturated") with cis geometry about widway in the chain; it makes up 55–80% of olive oil.
Elaidic acid is its trans isomer; it may be present in partially hydrogenated vegetable oils, and also occurs in the fat of the durian fruit (about 2%) and in milk fat (less than 0.1%).
Vaccenic acid is another trans acid that differs from elaidic only in the position of the double bond; it also occurs in milk fat (about 1-2%).
Nomenclature
Common fat names
Fats are usually named after their source (like butterfat, olive oil, cod liver oil, tail fat) or have traditional names of their own (like butter, lard, ghee, and margarine). Some of these names refer to products that contain substantial amounts of other components besides fats proper.
Chemical fatty acid names
In chemistry and biochemistry, dozens of saturated fatty acids and of hundreds of unsaturated ones have proper scientfic/technical names usually inspired by their source fats (butyric, caprylic, stearic, oleic, palmitic, and nervonic), but sometimes their discoverer (mead, osbond).
A triglyceride would then be named as an ester of those acids, such as "glyceryl 1,2-dioleate 3-palmitate".[6]
IUPAC
In the general chemical nomenclature developed by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of a fatty acid, derived from the name of the corresponding hydrocarbon, completely describes its structure, by specifying the number of carbons and the number and position of the double bonds. Thus, for example, oleic acid would be called "(9Z)-octadec-9-enoic acid", meaning that it has a 18 carbon chain ("octadec") with a carboxyl at one end ("oic") and a double bound at carbon 9 counting from the carboxyl ("9-en"), and that the configuration of the single bonds adjacent to that double bond is cis ("(9Z)") The IUPAC nomenclature can also handle branched chains and derivatives where hydrogen atoms are replaced by other chemical groups.
A triglyceride would then be named according to general ester rules as, for example, "propane-1,2,3-tryl 1,2-bis((9Z)-octadec-9-enoate) 3-(hexadecanoate)".
Fatty acid code
A notation specific for fatty acids with unbranched chain, that is as precise as the IUPAC one but easier to parse, is a code of the form "{N}:{D} cis-{CCC} trans-{TTT}", where {N} is the number of carbons (including the carboxyl one), {D} is the number of double bonds, {CCC} is a list of the positions of the cis double bonds, and {TTT} is a list of the postions of the trans bounds. Either list and the label is omitted if there are no bounds of that type.
Thus, for example, the codes for stearic, oleic, elaidic, and vaccenic acids would be "18:0", "18:1 cis-9", "18:1 trans-9", and "18:1 trans-11", respectively. The code for α-oleostearic acid, which is "(9E,11E,13Z)-octadeca-9,11,13-trienoic acid" in the IUPAC nomenclature, has the code "18:3 trans-9,11 cis-13"
Classification
By chain length
Fats can be classified according to the lengths of the carbon chains of their constituent fatty acids. Most chemical properties, such as melting point and acidity, vary gradually with this parameter, so there is no sharp division. Chemically, formic acid (1 carbon) and acetic acid (2 carbons) could be viewed as the shortest fatty acids; then triformin would be the simplest trigliceride. However, the terms "fatty acid" and "fat" are usually reserved for compounds with substantially longer chains.
A division commonly made in biochemistry and nutrition is:
- Short-chain fatty acid (SCFA) with less than six carbons (e. g. butyric acid).
- Medium-chain fatty acid (MCFA) with 6 to 12 carbons (e.g. capric acid).
- Long-chain fatty acids (LCFA) with 13 to 21 carbons (e.g. petroselinic acid).
- Very long chain fatty acids (VLCFA) with 22 or more carbons (e. g. cerotic acid with 26)
A triglyceride molecule may have fatty acid elements of different lengths, and a fat product will often be a mix of various triglycerides. Most fats found in food, whether vegetable or animal, are made up of medium to long-chain fatty acids, usually of equal or nearly equal length.
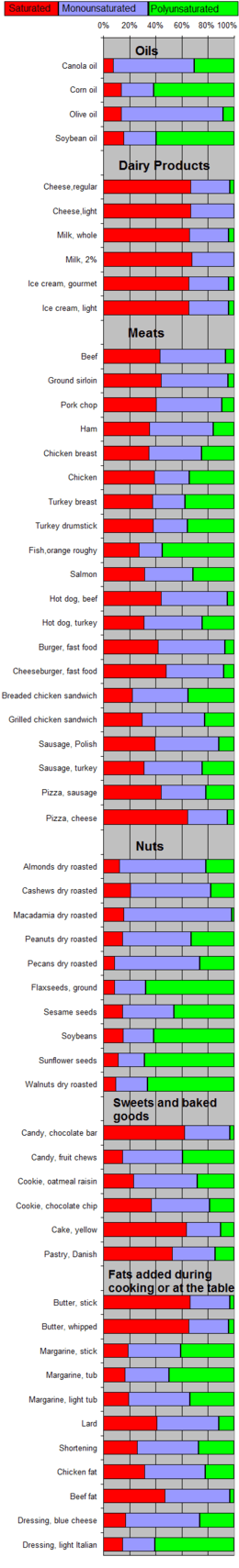
Saturated and unsaturated fats
For human nutrition, an important classification of fats is based on the number and position of double bonds in the constituent fatty acids. Saturated fat has a predominance of saturated fatty acids, without any double bonds, while unsaturated fat has predominantly unsaturated acids with double bonds.
Unsaturated fatty acids are further classified into "monounsaturated", with a single double bond, and "polyunsaturated" with two or more.
The molecules of saturated fats usually can arrange themselves more compactly, and thus are more likely to be solid at room temperature. For example, animal fats tallow and lard are high in saturated fatty acid content and are solids. Olive and linseed oils on the other hand are unsaturated and liquid. Unsaturated fats are prone to oxidation by air, which causes them to become rancid and inedible.
The double bonds in unsaturated fats can be converted into single bonds by reaction with hydrogen effected by a catalyst. This process, called hydrogenation, is used to turn vegetable vegetable oils into solid or semisolid vegetable fats like margarine, which can substitute for tallow and butter and (unlike unsaturated fats) can be stored indefinitely without becoming rancid. However, trans fats are generated during hydrogenation as contaminants created by an unwanted side reaction on the catalyst during partial hydrogenation.
Cis and trans fats
Another important classification of unsaturated fatty acids considers the cis-trans isomerism, the spatial arrangement of the single bonds adjacent to the double bonds. Most unsaturated fatty acids that occur in nature have those bonds in the cis ("same side") configuration.
Omega number
Another classification considers the position of double bonds, counted from the "ω" (omega) or "n" carbon atom at the end opposite to the carboxyl group. Thus, for example, alpha-linolenic acid is an omega-3 fatty acid because the 3rd carbon from that end is the first to have a double bond.
Biological importance
In humans and many animals, fats serve both as energy sources and as stores for energy in excess of what the body needs immediately. Each gram of fat when burned or metabolized releases about 9 food calories (37 kJ = 8.8 kcal).[7]
Fats are also sources of essential fatty acids, an important dietary requirement. Vitamins A, D, E, and K are fat-soluble, meaning they can only be digested, absorbed, and transported in conjunction with fats.
Fats play a vital role in maintaining healthy skin and hair, insulating body organs against shock, maintaining body temperature, and promoting healthy cell function. Fat also serves as a useful buffer against a host of diseases. When a particular substance, whether chemical or biotic, reaches unsafe levels in the bloodstream, the body can effectively dilute—or at least maintain equilibrium of—the offending substances by storing it in new fat tissue. This helps to protect vital organs, until such time as the offending substances can be metabolized or removed from the body by such means as excretion, urination, accidental or intentional bloodletting, sebum excretion, and hair growth.
Adipose tissue
In animals, adipose tissue, or fatty tissue is the body's means of storing metabolic energy over extended periods of time. Adipocytes (fat cells) store fat derived from the diet and from liver metabolism. Under energy stress these cells may degrade their stored fat to supply fatty acids and also glycerol to the circulation. These metabolic activities are regulated by several hormones (e.g., insulin, glucagon and epinephrine). Adipose tissue also secretes the hormone leptin.[8]
The location of the tissue determines its metabolic profile: visceral fat is located within the abdominal wall (i.e., beneath the wall of abdominal muscle) whereas subcutaneous fat is located beneath the skin (and includes fat that is located in the abdominal area beneath the skin but above the abdominal muscle wall). Visceral fat was recently discovered to be a significant producer of signaling chemicals (i.e., hormones), among which several are involved in inflammatory tissue responses. One of these is resistin which has been linked to obesity, insulin resistance, and Type 2 diabetes. This latter result is currently controversial, and there have been reputable studies supporting all sides on the issue.
Nutritional and health aspects
The benefits and risks of dietary fats have been the object of much study, and are still highly controversial topics.[9][10][11][12]
Saturated vs. unsaturated fats
Studies have found that replacing saturated fats with cis unsaturated fats in the diet reduces risk of cardiovascular disease. For example, a 2020 systematic review of randomized control trials by the Cochrane Library concluded: "Lifestyle advice to all those at risk of cardiovascular disease and to lower risk population groups should continue to include permanent reduction of dietary saturated fat and partial replacement by unsaturated fats."[13]
Cis vs. trans fats
Numerous studies have also found that consumption of trans fats increases risk of cardiovascular disease.[14][7] The Harvard School of Public Health advises that replacing trans fats and saturated fats with cis monounsaturated and polyunsaturated fats is beneficial for health.[15]
Essential fatty acids
There are two essential fatty acids (EFAs) in human nutrition: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).[14][7] Other lipids needed by the body can be synthesized from these and other fats.
Fat digestion and metabolism
Fats are broken down in the healthy body to release their constituents, glycerol and fatty acids. Glycerol itself can be converted to glucose by the liver and so become a source of energy. Fats and other lipids are broken down in the body by enzymes called lipases produced in the pancreas.
Many cell types can use either glucose or fatty acids as a source of energy for metabolism. In particular, heart and skeletal muscle prefer fatty acids. Despite long-standing assertions to the contrary, fatty acids can also be used as a source of fuel for brain cells through mitochondrial oxidation. [16]
See also
References
- Entry for "fat" in the online Merriam-Webster disctionary, sense 3.2. Accessed on 2020-08-09
- Thomas A. B. Sanders (2016): "The Role of Fats in Human Diet". Pages 1-20 of Functional Dietary Lipids. Woodhead/Elsevier, 332 pages. ISBN 978-1-78242-247-1doi:10.1016/B978-1-78242-247-1.00001-6
- "Macronutrients: the Importance of Carbohydrate, Protein, and Fat". McKinley Health Center. University of Illinois at Urbana–Champaign. Retrieved 20 September 2014.
- "Introduction to Energy Storage". Khan Academy.
- Anna Ohtera, Yusaku Miyamae, Naomi Nakai, Atsushi Kawachi, Kiyokazu Kawada, Junkyu Han, Hiroko Isoda, Mohamed Neffati, Toru Akita, Kazuhiro Maejima, Seiji Masuda, Taiho Kambe, Naoki Mori, Kazuhiro Irie, and Masaya Nagao (2013): "Identification of 6-octadecynoic acid from a methanol extract of Marrubium vulgare L. as a peroxisome proliferator-activated receptor γ agonist". Biochemical and Biophysical Research Communications, volume 440, issue 2, pages 204-209. doi:10.1016/j.bbrc.2013.09.003
- N. Koeniger and H. J. Veith (1983): "Glyceryl-1,2-dioleate-3-palmitate, a brood pheromone of the honey bee (Apis mellifera L.)". Experientia, volume 39, pages 1051–1052 doi:10.1007/BF01989801
- Government of the United Kingdom (1996): "Schedule 7: Nutrition labelling". In Food Labelling Regulations 1996'. Accessed on 2020-08-09.
- "The human proteome in adipose - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2017-09-12.
- Rebecca J. Donatelle (2005): Health, the Basics, 6th edition. Pearson Education, San Francisco; ISBN 978-0-13-120687-8
- Frank B. Hu, JoAnn E. Manson, and Walter C. Willett (2001): "Types of dietary fat and risk of coronary heart disease: A critical review". Journal of the American College of Nutrition, volume 20, issue 1, pages 5-19. doi:10.1080/07315724.2001.10719008
- Lee Hooper, Carolyn D. Summerbell, Julian P. T. Higgins, Rachel L. Thompson, Nigel E. Capps, George Davey Smith, Rudolph A. Riemersma, and Shah Ebrahim (2001): "Dietary fat intake and prevention of cardiovascular disease: systematic review". The BMJ, volume 322, pages 757-. doi:10.1136/bmj.322.7289.757
- George A. Bray, Sahasporn Paeratakul, Barry M. Popkin (2004): "Dietary fat and obesity: a review of animal, clinical and epidemiological studies". Physiology & Behavior, volume 83, issue 4, pages 549-555. doi:10.1016/j.physbeh.2004.08.039
- Lee Hooper, Nicole Martin, Oluseyi F. Jimoh, Christian Kirk, Eve Foster, and Asmaa S. Adbelhamid (2020): "Reduction in saturated fat intake for cardiovascular disease". The Cochrane Database of Systematic Reviews, volume 5, article CD011737. PMID 32428300 doi:10.1002/14651858.CD011737.pub2
- Dariush Mozaffarian, Martijn B. Katan, Alberto Ascherio, Meir J. Stampfer, and Walter C. Willett (2006): "Trans fatty acids and cardiovascular disease". New England Journal of Medicine, volume 354, issue 15, pages 1601–1613. doi:10.1056/NEJMra054035 PMID 16611951
- "Fats and Cholesterol", Harvard School of Public Health. Retrieved 02-11-16.
- Panov, Alexander; Orynbayeva, Zulfiya; Vavilin, Valentin; Lyakhovich, Vyacheslav. Baranova, Ancha (ed.). "Fatty Acids in Energy Metabolism of the Central Nervous System". BioMed Research International. Hindawa. 2014 (The Roads to Mitochondrial Dysfunction).
Further reading
| Wikibooks Cookbook has a recipe/module on |
| Look up Fat in Wiktionary, the free dictionary. |
- Hayes, K.C. (May 2005). Dietary fat and blood lipids. Retrieved March 10, 2011.