Terbutaline
Terbutaline, sold under the brand name Bricanyl among others, is a β2 adrenergic receptor agonist, used as a "reliever" inhaler in the management of asthma symptoms and as a tocolytic (anti-contraction medication) to delay preterm labor for up to 48 hours. This time can then be used to administer steroid injections to the mother which help fetal lung maturity and reduce complications of prematurity.[1] It should not be used to prevent preterm labor or delay labor more than 48–72 hours. In February 2011, the Food and Drug Administration began requiring a black box warning on the drug's label. Pregnant women should not be given injections of the drug terbutaline for the prevention of preterm labor or for long-term (beyond 48–72 hours) management of preterm labor, and should not be given oral terbutaline for any type of prevention or treatment of preterm labor "due to the potential for serious internal heart problems and death."[2][3]
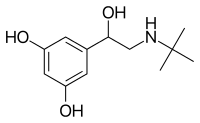 | |
 Terbutaline (top), and (R)-(−)-terbutaline (bottom) | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682144 |
| Pregnancy category |
|
| Routes of administration | Oral (tablets, oral solution), inhalational (DPI, nebulizer solution), SQ |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 25% |
| Metabolism | GI tract (oral), liver; CYP450: unknown |
| Elimination half-life | 11-16 hours |
| Excretion | urine 90% (60% unchanged), bile/faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.244 |
| Chemical and physical data | |
| Formula | C12H19NO3 |
| Molar mass | 225.288 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
It was patented in 1966 and came into medical use in 1970.[4]
Medical uses
Terbutaline is used as a fast-acting bronchodilator (often used as a short-term asthma treatment) and as a tocolytic[5] to delay premature labor. The inhaled form of terbutaline starts working within 15 minutes and can last up to 6 hours.
Terbutaline as a treatment for premature labor is an off-label use not approved by the FDA. It is a pregnancy category C medication and is routinely prescribed to stop contractions. After successful intravenous tocolysis, little evidence exists that oral terbutaline is effective.[6] However, following uterine inversion in the third stage of labor, terbutaline (or either Halothane or magnesium sulfate) can be used to relax the uterus if necessary prior to uterine replacement.
Side effects
- Adult — tachycardia, anxiety, nervousness, tremors, headache, hyperglycemia, hypokalemia, hypotension and, rarely, pulmonary edema.[7]
- Fetal — tachycardia and hypoglycemia.[8]
Pharmacology
The tertiary butyl group in terbutaline makes it more selective for β2 receptors. Since there is no hydroxy group on position 4 of the benzene ring, the molecule is less susceptible to metabolism by the enzyme catechol-O-methyl transferase.[9]
Chemistry
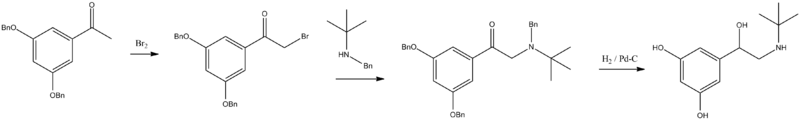
Terbutaline is synthesized by brominating 3,5-dibenzyloxyacetophenone into 3,5-dibenzyloxybromoacetophenone, which is reacted with N-benzyl-N-tert-butylamine, giving a ketone intermediate. Reduction of this product with H₂ over Pd/C leads to terbutaline.[10][11][12]
Society and culture
Athletics
As with all β2-adrenergic receptor agonists, terbutaline is on the World Anti-Doping Agency's list of prohibited drugs, except when administered by inhalation and a Therapeutic Use Exemption (TUE) has been obtained in advance.
Brand names
Brand names include Bronclyn, Brethine, Bricanyl, Brethaire, or Terbulin.
References
- WHO. "Antenatal administration of corticosteroids for women at risk of preterm birth". WHO. Retrieved 2013-03-25.
- "Most Popular E-mail Newsletter". USA Today. 18 February 2011.
- "Archived copy". Archived from the original on 2015-09-21. Retrieved 2015-09-13.CS1 maint: archived copy as title (link)
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 542. ISBN 9783527607495.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR (September 2008). "Nifedipine versus terbutaline for tocolysis in external cephalic version". Int J Gynaecol Obstet. 102 (3): 263–6. doi:10.1016/j.ijgo.2008.04.010. PMID 18554601.
- Goldenberg, RL (November 2002). "High-Risk Pregnancy Series: An Expert's View". Obstetrics & Gynecology. 100 (5): 1020–1037. doi:10.1016/S0029-7844(02)02212-3. Archived from the original on 2011-07-20.
- Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 7. ISBN 1-59541-101-1.
- , 5 Minute Consult (Original Source: UpToDate "Terbutaline: Drug information").
- "Medicinal Chemistry of Adrenergics and Cholinergics". Archived from the original on 2010-11-04.
- Draco Lunds Farmacevtiska Aktiebolag, GB 1199630 (1967)
- Draco Lunds Farmacevtiska Aktiebolag, BE 704932 (1968)
- L. A. Svensson, I. K. Wetterlin, U.S. Patent 3,937,838 (1976)
- F. v. Bruchhausen, G. Dannhardt, S. Ebel, A. W. Frahm, E. Hackenthal, U. Holzgrabe (Hrsg.): Hagers Handbuch der Pharmazeutischen Praxis: Band 9: Stoffe P-Z, Springer Verlag, Berlin, Aufl. 5, 2014, S. 804, ISBN 978-3-642-63389-8.
-Terbutalin_Structural_Formula_V1.svg.png)
-Terbutalin_Structural_Formula_V1.svg.png)