Alzheimer's disease
Alzheimer's disease (AD), also referred to simply as Alzheimer's, is a chronic neurodegenerative disease that usually starts slowly and gradually worsens over time.[1][2] It is the cause of 60–70% of cases of dementia.[1][2] The most common early symptom is difficulty in remembering recent events.[1] As the disease advances, symptoms can include problems with language, disorientation (including easily getting lost), mood swings, loss of motivation, not managing self-care, and behavioural issues.[1][2] As a person's condition declines, they often withdraw from family and society.[1] Gradually, bodily functions are lost, ultimately leading to death.[10] Although the speed of progression can vary, the typical life expectancy following diagnosis is three to nine years.[7][11]
| Alzheimer's disease | |
|---|---|
| Other names | Alzheimer disease, Alzheimer's |
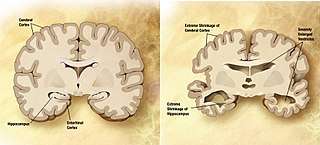 | |
| Comparison of a normal aged brain (left) and the brain of a person with Alzheimer's (right). Characteristics that separate the two are pointed out. | |
| Pronunciation |
|
| Specialty | Neurology |
| Symptoms | Difficulty in remembering recent events, problems with language, disorientation, mood swings[1][2] |
| Usual onset | Over 65 years old[3] |
| Duration | Long term[2] |
| Causes | Poorly understood[1] |
| Risk factors | Genetics, head injuries, depression, hypertension[1][4] |
| Diagnostic method | Based on symptoms and cognitive testing after ruling out other possible causes[5] |
| Differential diagnosis | Normal aging[1] |
| Medication | Acetylcholinesterase inhibitors, NMDA receptor antagonists (small benefit)[6] |
| Prognosis | Life expectancy 3–9 years[7] |
| Frequency | 29.8 million (2015)[2][8] |
| Deaths | 1.9 million (2015)[9] |
The cause of Alzheimer's disease is poorly understood.[1] About 70% of the risk is believed to be inherited from a person's parents, with many genes usually involved.[4] Other risk factors include a history of head injuries, depression, and hypertension.[1] The disease process is associated with plaques and neurofibrillary tangles in the brain.[4] A probable diagnosis is based on the history of the illness and cognitive testing with medical imaging and blood tests to rule out other possible causes.[5] Initial symptoms are often mistaken for normal ageing.[1] Examination of brain tissue is needed for a definite diagnosis.[4] Mental and physical exercise, and avoiding obesity may decrease the risk of AD; however, evidence to support these recommendations is weak.[4][12] There are no medications or supplements that have been shown to decrease risk.[13]
No treatments stop or reverse its progression, though some may temporarily improve symptoms.[2] Affected people increasingly rely on others for assistance, often placing a burden on the caregiver.[14] The pressures can include social, psychological, physical, and economic elements.[14] Exercise programs may be beneficial with respect to activities of daily living and can potentially improve outcomes.[15] Behavioural problems or psychosis due to dementia are often treated with antipsychotics, but this is not usually recommended, as there is little benefit and an increased risk of early death.[16][17]
In 2015, there were approximately 29.8 million people worldwide with AD.[2][8] It most often begins in people over 65 years of age, although 4–5% of cases are early-onset Alzheimer's.[3] It affects about 6% of people 65 years and older.[1] In 2015, dementia resulted in about 1.9 million deaths.[9] It was first described by, and later named after, German psychiatrist and pathologist Alois Alzheimer in 1906.[18] In developed countries, AD is one of the most financially costly diseases.[19][20]
Signs and symptoms
- Effects of ageing on memory but not AD
- Forgetting things occasionally
- Misplacing items sometimes
- Minor short-term memory loss
- Not remembering exact details
- Early stage Alzheimer's
- Not remembering episodes of forgetfulness
- Forgets names of family or friends
- Changes may only be noticed by close friends or relatives
- Some confusion in situations outside the familiar
- Middle stage Alzheimer's
- Greater difficulty remembering recently learned information
- Deepening confusion in many circumstances
- Problems with sleep
- Trouble determining their location
- Late stage Alzheimer's
- Poor ability to think
- Problems speaking
- Repeats same conversations
- More abusive, anxious, or paranoid
The disease course is divided into four stages, with a progressive pattern of cognitive and functional impairment.
Pre-dementia
The first symptoms are often mistakenly attributed to ageing or stress.[22] Detailed neuropsychological testing can reveal mild cognitive difficulties up to eight years before a person fulfills the clinical criteria for diagnosis of AD.[23] These early symptoms can affect the most complex activities of daily living.[24] The most noticeable deficit is short term memory loss, which shows up as difficulty in remembering recently learned facts and inability to acquire new information.[23][25]
Subtle problems with the executive functions of attentiveness, planning, flexibility, and abstract thinking, or impairments in semantic memory (memory of meanings, and concept relationships) can also be symptomatic of the early stages of AD.[23] Apathy can be observed at this stage, and remains the most persistent neuropsychiatric symptom throughout the course of the disease.[26] Depressive symptoms, irritability and reduced awareness of subtle memory difficulties are also common.[27] The preclinical stage of the disease has also been termed mild cognitive impairment (MCI).[25] This is often found to be a transitional stage between normal ageing and dementia. MCI can present with a variety of symptoms, and when memory loss is the predominant symptom, it is termed "amnestic MCI" and is frequently seen as a prodromal stage of Alzheimer's disease.[28]
Early
In people with AD, the increasing impairment of learning and memory eventually leads to a definitive diagnosis. In a small percentage, difficulties with language, executive functions, perception (agnosia), or execution of movements (apraxia) are more prominent than memory problems.[29] AD does not affect all memory capacities equally. Older memories of the person's life (episodic memory), facts learned (semantic memory), and implicit memory (the memory of the body on how to do things, such as using a fork to eat or how to drink from a glass) are affected to a lesser degree than new facts or memories.[30][31]
Language problems are mainly characterised by a shrinking vocabulary and decreased word fluency, leading to a general impoverishment of oral and written language.[29][32] In this stage, the person with Alzheimer's is usually capable of communicating basic ideas adequately.[29][32][33] While performing fine motor tasks such as writing, drawing, or dressing, certain movement coordination and planning difficulties (apraxia) may be present, but they are commonly unnoticed.[29] As the disease progresses, people with AD can often continue to perform many tasks independently, but may need assistance or supervision with the most cognitively demanding activities.[29]
Moderate
Progressive deterioration eventually hinders independence, with subjects being unable to perform most common activities of daily living.[29] Speech difficulties become evident due to an inability to recall vocabulary, which leads to frequent incorrect word substitutions (paraphasias). Reading and writing skills are also progressively lost.[29][33] Complex motor sequences become less coordinated as time passes and AD progresses, so the risk of falling increases.[29] During this phase, memory problems worsen, and the person may fail to recognise close relatives.[29] Long-term memory, which was previously intact, becomes impaired.[29]
Behavioural and neuropsychiatric changes become more prevalent. Common manifestations are wandering, irritability and labile affect, leading to crying, outbursts of unpremeditated aggression, or resistance to caregiving.[29] Sundowning can also appear.[34] Approximately 30% of people with AD develop illusionary misidentifications and other delusional symptoms.[29] Subjects also lose insight of their disease process and limitations (anosognosia).[29] Urinary incontinence can develop.[29] These symptoms create stress for relatives and carers, which can be reduced by moving the person from home care to other long-term care facilities.[29][35]
Advanced
During the final stages, the patient is completely dependent upon caregivers.[29] Language is reduced to simple phrases or even single words, eventually leading to complete loss of speech.[29][33] Despite the loss of verbal language abilities, people can often understand and return emotional signals. Although aggressiveness can still be present, extreme apathy and exhaustion are much more common symptoms. People with Alzheimer's disease will ultimately not be able to perform even the simplest tasks independently; muscle mass and mobility deteriorates to the point where they are bedridden and unable to feed themselves. The cause of death is usually an external factor, such as infection of pressure ulcers or pneumonia, not the disease itself.[29]
Causes
The cause for most Alzheimer's cases is still mostly unknown except for 1% to 5% of cases where genetic differences have been identified.[36][37] Several competing hypotheses exist trying to explain the cause of the disease.
Genetic
The genetic heritability of Alzheimer's disease (and memory components thereof), based on reviews of twin and family studies, ranges from 49% to 79%.[38] Around 0.1% of the cases are familial forms of autosomal (not sex-linked) dominant inheritance, which have an onset before age 65.[39] This form of the disease is known as early onset familial Alzheimer's disease. Most of autosomal dominant familial AD can be attributed to mutations in one of three genes: those encoding amyloid precursor protein (APP) and presenilins 1 and 2.[40] Most mutations in the APP and presenilin genes increase the production of a small protein called Aβ42, which is the main component of senile plaques.[41] Some of the mutations merely alter the ratio between Aβ42 and the other major forms—particularly Aβ40—without increasing Aβ42 levels.[42] Two other genes associated with autosomal dominant Alzheimer's disease are ABCA7 and SORL1.[43]
Most cases of Alzheimer's disease do not exhibit autosomal-dominant inheritance and are termed sporadic AD, in which environmental and genetic differences may act as risk factors. The best known genetic risk factor is the inheritance of the ε4 allele of the apolipoprotein E (APOE).[44][45] Between 40 and 80% of people with AD possess at least one APOEε4 allele.[45] The APOEε4 allele increases the risk of the disease by three times in heterozygotes and by 15 times in homozygotes.[39] Like many human diseases, environmental effects and genetic modifiers result in incomplete penetrance. For example, certain Nigerian populations do not show the relationship between dose of APOEε4 and incidence or age-of-onset for Alzheimer's disease seen in other human populations.[46][47] Early attempts to screen up to 400 candidate genes for association with late-onset sporadic AD (LOAD) resulted in a low yield.[39][40] More recent genome-wide association studies (GWAS) have found 19 areas in genes that appear to affect the risk.[48] These genes include: CASS4, CELF1, FERMT2, HLA-DRB5, INPP5D, MEF2C, NME8, PTK2B, SORL1, ZCWPW1, SLC24A4, CLU, PICALM, CR1, BIN1, MS4A, ABCA7, EPHA1, and CD2AP.[48]
Alleles in the TREM2 gene have been associated with a 3 to 5 times higher risk of developing Alzheimer's disease.[49][50] A suggested mechanism of action is that in some variants in TREM2, white blood cells in the brain are no longer able to control the amount of beta amyloid present. Many single-nucleotide polymorphisms (SNPs) are associated with Alzheimer's, with a 2018 study adding 30 SNPs by differentiating AD into 6 categories, including memory, language, visuospatial, and executive functioning.[51]
Cholinergic hypothesis
The oldest hypothesis, on which most currently available drug therapies are based, is the cholinergic hypothesis,[52] which proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine. The cholinergic hypothesis has not maintained widespread support, largely because medications intended to treat acetylcholine deficiency have not been very effective.[53]
Amyloid hypothesis
In 1991, the amyloid hypothesis postulated that extracellular amyloid beta (Aβ) deposits are the fundamental cause of the disease.[54][55] Support for this postulate comes from the location of the gene for the amyloid precursor protein (APP) on chromosome 21, together with the fact that people with trisomy 21 (Down Syndrome) who have an extra gene copy almost universally exhibit at least the earliest symptoms of AD by 40 years of age.[56][57] Also, a specific isoform of apolipoprotein, APOE4, is a major genetic risk factor for AD. While apolipoproteins enhance the breakdown of beta amyloid, some isoforms are not very effective at this task (such as APOE4), leading to excess amyloid buildup in the brain.[58] Further evidence comes from the finding that transgenic mice that express a mutant form of the human APP gene develop fibrillar amyloid plaques and Alzheimer's-like brain pathology with spatial learning deficits.[59]
An experimental vaccine was found to clear the amyloid plaques in early human trials, but it did not have any significant effect on dementia.[60] Researchers have been led to suspect non-plaque Aβ oligomers (aggregates of many monomers) as the primary pathogenic form of Aβ. These toxic oligomers, also referred to as amyloid-derived diffusible ligands (ADDLs), bind to a surface receptor on neurons and change the structure of the synapse, thereby disrupting neuronal communication.[61] One receptor for Aβ oligomers may be the prion protein, the same protein that has been linked to mad cow disease and the related human condition, Creutzfeldt–Jakob disease, thus potentially linking the underlying mechanism of these neurodegenerative disorders with that of Alzheimer's disease.[62]
In 2009, this hypothesis was updated, suggesting that a close relative of the beta-amyloid protein, and not necessarily the beta-amyloid itself, may be a major culprit in the disease. The hypothesis holds that an amyloid-related mechanism that prunes neuronal connections in the brain in the fast-growth phase of early life may be triggered by ageing-related processes in later life to cause the neuronal withering of Alzheimer's disease.[63] N-APP, a fragment of APP from the peptide's N-terminus, is adjacent to beta-amyloid and is cleaved from APP by one of the same enzymes. N-APP triggers the self-destruct pathway by binding to a neuronal receptor called death receptor 6 (DR6, also known as TNFRSF21).[63] DR6 is highly expressed in the human brain regions most affected by Alzheimer's, so it is possible that the N-APP/DR6 pathway might be hijacked in the ageing brain to cause damage. In this model, beta-amyloid plays a complementary role, by depressing synaptic function.
Osaka mutation
A Japanese pedigree of familial Alzheimer's disease was found to be associated with a deletion mutation of codon 693 of APP.[64] This mutation and its association with Alzheimer's disease was first reported in 2008.[65] This mutation is known as the Osaka mutation. Only homozygotes with this mutation develop Alzheimer's disease. This mutation accelerates Aβ oligomerization but the proteins do not form amyloid fibrils suggesting that it is the Aβ oligomerization rather than the fibrils that may be the cause of this disease. Mice expressing this mutation have all usual pathologies of Alzheimer's disease.
Tau hypothesis
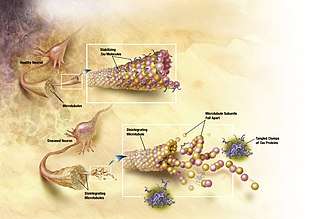
The tau hypothesis proposes that tau protein abnormalities initiate the disease cascade.[55] In this model, hyperphosphorylated tau begins to pair with other threads of tau. Eventually, they form neurofibrillary tangles inside nerve cell bodies.[66] When this occurs, the microtubules disintegrate, destroying the structure of the cell's cytoskeleton which collapses the neuron's transport system.[67] This may result first in malfunctions in biochemical communication between neurons and later in the death of the cells.[68]
Other hypotheses
An inflammatory hypothesis is that AD is caused due to a self-perpetuating progressive inflammation in the brain culminating in neurodegeneration.[69] A possible role of chronic periodontal infection[69] and the gut microbiota has been suggested.[70]
A neurovascular hypothesis has been proposed which states that poor functioning of the blood–brain barrier may be involved.[71] Spirochete infections have also been linked to dementia.[72][73]
The cellular homeostasis of biometals such as ionic copper, iron, and zinc is disrupted in AD, though it remains unclear whether this is produced by or causes the changes in proteins. These ions affect and are affected by tau, APP, and APOE,[74] and their dysregulation may cause oxidative stress that may contribute to the pathology.[75][76][77][78][79] The quality of some of these studies has been criticised,[80][81] and the link remains controversial.[82] The majority of researchers do not support a causal connection with aluminium.[81]
Smoking is a significant AD risk factor.[83] Systemic markers of the innate immune system are risk factors for late-onset AD.[84]
There is tentative evidence that exposure to air pollution may be a contributing factor to the development of Alzheimer's disease.[85]
One hypothesis posits that dysfunction of oligodendrocytes and their associated myelin during aging contributes to axon damage, which then causes amyloid production and tau hyper-phosphorylation as a side effect.[86][87]
Retrogenesis is a medical hypothesis about the development and progress of Alzheimer's disease proposed by Barry Reisberg in the 1980s.[88] The hypothesis is that just as the fetus goes through a process of neurodevelopment beginning with neurulation and ending with myelination, the brains of people with AD go through a reverse neurodegeneration process starting with demyelination and death of axons (white matter) and ending with the death of grey matter.[89] Likewise the hypothesis is, that as infants go through states of cognitive development, people with AD go through the reverse process of progressive cognitive impairment.[88] Reisberg developed the caregiving assessment tool known as "FAST" (Functional Assessment Staging Tool) which he says allows those caring for people with AD to identify the stages of disease progression and that provides advice about the kind of care needed at each stage.[88][90]
The association with celiac disease is unclear, with a 2019 study finding no increase in dementia overall in those with CD, while a 2018 review found an association with several types of dementia including AD.[91][92]
Pathophysiology
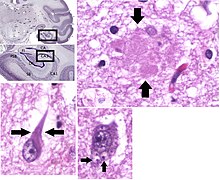
Neuropathology
Alzheimer's disease is characterised by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.[93] Degeneration is also present in brainstem nuclei like the locus coeruleus.[94] Studies using MRI and PET have documented reductions in the size of specific brain regions in people with AD as they progressed from mild cognitive impairment to Alzheimer's disease, and in comparison with similar images from healthy older adults.[95][96]
Both amyloid plaques and neurofibrillary tangles are clearly visible by microscopy in brains of those afflicted by AD,[97] especially in the hippocampus.[98] Plaques are dense, mostly insoluble deposits of beta-amyloid peptide and cellular material outside and around neurons. Tangles (neurofibrillary tangles) are aggregates of the microtubule-associated protein tau which has become hyperphosphorylated and accumulate inside the cells themselves. Although many older individuals develop some plaques and tangles as a consequence of ageing, the brains of people with AD have a greater number of them in specific brain regions such as the temporal lobe.[99] Lewy bodies are not rare in the brains of people with AD.[100]
Biochemistry
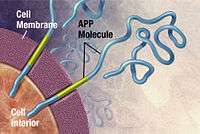
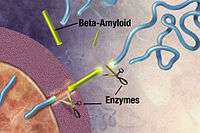
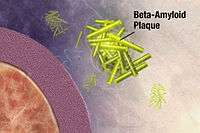
Alzheimer's disease has been identified as a protein misfolding disease (proteopathy), caused by plaque accumulation of abnormally folded amyloid beta protein and tau protein in the brain.[101] Plaques are made up of small peptides, 39–43 amino acids in length, called amyloid beta (Aβ). Aβ is a fragment from the larger amyloid precursor protein (APP). APP is a transmembrane protein that penetrates through the neuron's membrane. APP is critical to neuron growth, survival, and post-injury repair.[102][103] In Alzheimer's disease, gamma secretase and beta secretase act together in a proteolytic process which causes APP to be divided into smaller fragments.[104] One of these fragments gives rise to fibrils of amyloid beta, which then form clumps that deposit outside neurons in dense formations known as senile plaques.[97][105]
AD is also considered a tauopathy due to abnormal aggregation of the tau protein. Every neuron has a cytoskeleton, an internal support structure partly made up of structures called microtubules. These microtubules act like tracks, guiding nutrients and molecules from the body of the cell to the ends of the axon and back. A protein called tau stabilises the microtubules when phosphorylated, and is therefore called a microtubule-associated protein. In AD, tau undergoes chemical changes, becoming hyperphosphorylated; it then begins to pair with other threads, creating neurofibrillary tangles and disintegrating the neuron's transport system.[106] Pathogenic tau can also cause neuronal death through transposable element dysregulation.[107]
Disease mechanism
Exactly how disturbances of production and aggregation of the beta-amyloid peptide give rise to the pathology of AD is not known.[108][109] The amyloid hypothesis traditionally points to the accumulation of beta-amyloid peptides as the central event triggering neuron degeneration. Accumulation of aggregated amyloid fibrils, which are believed to be the toxic form of the protein responsible for disrupting the cell's calcium ion homeostasis, induces programmed cell death (apoptosis).[110] It is also known that Aβ selectively builds up in the mitochondria in the cells of Alzheimer's-affected brains, and it also inhibits certain enzyme functions and the utilisation of glucose by neurons.[111]
Various inflammatory processes and cytokines may also have a role in the pathology of Alzheimer's disease. Inflammation is a general marker of tissue damage in any disease, and may be either secondary to tissue damage in AD or a marker of an immunological response.[112] There is increasing evidence of a strong interaction between the neurons and the immunological mechanisms in the brain. Obesity and systemic inflammation may interfere with immunological processes which promote disease progression.[113]
Alterations in the distribution of different neurotrophic factors and in the expression of their receptors such as the brain-derived neurotrophic factor (BDNF) have been described in AD.[114][115]
Diagnosis
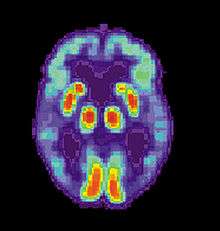
Alzheimer's disease is usually diagnosed based on the person's medical history, history from relatives, and behavioural observations. The presence of characteristic neurological and neuropsychological features and the absence of alternative conditions is supportive.[116][117] Advanced medical imaging with computed tomography (CT) or magnetic resonance imaging (MRI), and with single-photon emission computed tomography (SPECT) or positron emission tomography (PET) can be used to help exclude other cerebral pathology or subtypes of dementia.[118] Moreover, it may predict conversion from prodromal stages (mild cognitive impairment) to Alzheimer's disease.[119]
Assessment of intellectual functioning including memory testing can further characterise the state of the disease.[22] Medical organisations have created diagnostic criteria to ease and standardise the diagnostic process for practising physicians. The diagnosis can be confirmed with very high accuracy post-mortem when brain material is available and can be examined histologically.[120]
Criteria
The National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA, now known as the Alzheimer's Association) established the most commonly used NINCDS-ADRDA Alzheimer's Criteria for diagnosis in 1984,[120] extensively updated in 2007.[121] These criteria require that the presence of cognitive impairment, and a suspected dementia syndrome, be confirmed by neuropsychological testing for a clinical diagnosis of possible or probable AD. A histopathologic confirmation including a microscopic examination of brain tissue is required for a definitive diagnosis. Good statistical reliability and validity have been shown between the diagnostic criteria and definitive histopathological confirmation.[122] Eight intellectual domains are most commonly impaired in AD—memory, language, perceptual skills, attention, motor skills, orientation, problem solving and executive functional abilities. These domains are equivalent to the NINCDS-ADRDA Alzheimer's Criteria as listed in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) published by the American Psychiatric Association.[123][124]
Techniques

Neuropsychological tests such as the mini–mental state examination (MMSE) are widely used to evaluate the cognitive impairments needed for diagnosis. More comprehensive test arrays are necessary for high reliability of results, particularly in the earliest stages of the disease.[125][126] Neurological examination in early AD will usually provide normal results, except for obvious cognitive impairment, which may not differ from that resulting from other diseases processes, including other causes of dementia.
Further neurological examinations are crucial in the differential diagnosis of AD and other diseases.[22] Interviews with family members are also utilised in the assessment of the disease. Caregivers can supply important information on the daily living abilities, as well as on the decrease, over time, of the person's mental function.[127] A caregiver's viewpoint is particularly important, since a person with AD is commonly unaware of his own deficits.[128] Many times, families also have difficulties in the detection of initial dementia symptoms and may not communicate accurate information to a physician.[129]
Supplemental testing provides extra information on some features of the disease or is used to rule out other diagnoses. Blood tests can identify other causes for dementia than AD[22]—causes which may, in rare cases, be reversible.[130] It is common to perform thyroid function tests, assess B12, rule out syphilis, rule out metabolic problems (including tests for kidney function, electrolyte levels and for diabetes), assess levels of heavy metals (e.g., lead, mercury) and anaemia. (It is also necessary to rule out delirium).
Psychological tests for depression are employed, since depression can either be concurrent with AD (see Depression of Alzheimer disease), an early sign of cognitive impairment,[131] or even the cause.[132][133]
Due to low accuracy, the C-PIB-PET scan is not recommended to be used as an early diagnostic tool or for predicting the development of Alzheimer's disease when people show signs of mild cognitive impairment (MCI).[134] The use of ¹⁸F-FDG PET scans, as a single test, to identify people who may develop Alzheimer's disease is also not supported by evidence.[135]
Prevention

There is no definitive evidence to support that any particular measure is effective in preventing AD.[13] Global studies of measures to prevent or delay the onset of AD have often produced inconsistent results. Epidemiological studies have proposed relationships between certain modifiable factors, such as diet, cardiovascular risk, pharmaceutical products, or intellectual activities among others, and a population's likelihood of developing AD. Only further research, including clinical trials, will reveal whether these factors can help to prevent AD.[13]
Medication
Cardiovascular risk factors, such as hypercholesterolaemia, hypertension, diabetes, and smoking, are associated with a higher risk of onset and worsened course of AD.[136][137] Blood pressure medications may decrease the risk.[138] Statins, which lower cholesterol however, have not been effective in preventing or improving the course of the disease.[139][140][141]
Long-term usage of non-steroidal anti-inflammatory drugs (NSAIDs) were thought in 2007 to be associated with a reduced likelihood of developing AD.[142] Evidence also suggested the notion that NSAIDs could reduce inflammation related to amyloid plaques, but trials were suspended due to high adverse events.[13] No prevention trial has been completed.[13] They do not appear to be useful as a treatment, but as of 2011 were thought to be candidates as presymptomatic preventives.[143] Hormone replacement therapy in menopause, although previously used, may increase the risk of dementia.[144]
Lifestyle
People who engage in intellectual activities such as reading, playing board games, completing crossword puzzles, playing musical instruments, or regular social interaction show a reduced risk for Alzheimer's disease.[145] This is compatible with the cognitive reserve theory, which states that some life experiences result in more efficient neural functioning providing the individual a cognitive reserve that delays the onset of dementia manifestations.[145] Education delays the onset of AD syndrome without changing the duration of the disease.[146] Learning a second language even later in life seems to delay the onset of Alzheimer's disease.[147] Physical activity is also associated with a reduced risk of AD.[146] Physical exercise is associated with decreased rate of dementia.[148] Physical exercise is also effective in reducing symptom severity in those with Alzheimer's disease.[149]
Diet
People who maintain a healthy, Japanese, or Mediterranean diet have a reduced risk of AD.[150] A Mediterranean diet may improve outcomes in those with the disease.[151] Those who eat a diet high in saturated fats and simple carbohydrates (mono- and disaccharide) have a higher risk.[152] The Mediterranean diet's beneficial cardiovascular effect has been proposed as the mechanism of action.[153]
Conclusions on dietary components have at times been difficult to ascertain as results have differed between population-based studies and randomised controlled trials.[150] There is limited evidence that light to moderate use of alcohol, particularly red wine, is associated with lower risk of AD.[150] There is tentative evidence that caffeine may be protective.[154] A number of foods high in flavonoids such as cocoa, red wine, and tea may decrease the risk of AD.[155][156]
Reviews on the use of vitamins and minerals have not found enough consistent evidence to recommend them. This includes vitamin A,[157][158] C,[159][160] the alpha-tocopherol form of vitamin E,[161] selenium,[162] zinc,[163][164] and folic acid with or without vitamin B12.[165] Evidence from one randomized controlled trial indicated that the alpha-tocopherol form of vitamin E may slow cognitive decline, this evidence was judged to be "moderate" in quality.[161] Trials examining folic acid (B9) and other B vitamins failed to show any significant association with cognitive decline.[166] Omega-3 fatty acid supplements from plants and fish, and dietary docosahexaenoic acid (DHA), do not appear to benefit people with mild to moderate Alzheimer's disease.[167][168]
Curcumin as of 2010 had not shown benefit in people even though there is tentative evidence in animals.[169] There was inconsistent and unconvincing evidence that ginkgo has any positive effect on cognitive impairment and dementia.[170] As of 2008 there was no concrete evidence that cannabinoids are effective in improving the symptoms of AD or dementia;[171] however, some research into endocannabinoids looked promising.[172]
Management
There is no cure for Alzheimer's disease; available treatments offer relatively small symptomatic benefit but remain palliative in nature. Current treatments can be divided into pharmaceutical, psychosocial and caregiving.
Medications
Five medications are currently used to treat the cognitive problems of AD: four are acetylcholinesterase inhibitors (tacrine, rivastigmine, galantamine and donepezil) and the other (memantine) is an NMDA receptor antagonist. The benefit from their use is small.[173][174][175] No medication has been clearly shown to delay or halt the progression of the disease.
Reduction in the activity of the cholinergic neurons is a well-known feature of Alzheimer's disease.[176] Acetylcholinesterase inhibitors are employed to reduce the rate at which acetylcholine (ACh) is broken down, thereby increasing the concentration of ACh in the brain and combating the loss of ACh caused by the death of cholinergic neurons.[177] There is evidence for the efficacy of these medications in mild to moderate Alzheimer's disease,[178][174][173] and some evidence for their use in the advanced stage.[173] The use of these drugs in mild cognitive impairment has not shown any effect in a delay of the onset of AD.[179] The most common side effects are nausea and vomiting, both of which are linked to cholinergic excess. These side effects arise in approximately 10–20% of users, are mild to moderate in severity, and can be managed by slowly adjusting medication doses.[180] Less common secondary effects include muscle cramps, decreased heart rate (bradycardia), decreased appetite and weight, and increased gastric acid production.[178]
Glutamate is an excitatory neurotransmitter of the nervous system, although excessive amounts in the brain can lead to cell death through a process called excitotoxicity which consists of the overstimulation of glutamate receptors. Excitotoxicity occurs not only in Alzheimer's disease, but also in other neurological diseases such as Parkinson's disease and multiple sclerosis.[181] Memantine is a noncompetitive NMDA receptor antagonist first used as an anti-influenza agent. It acts on the glutamatergic system by blocking NMDA receptors and inhibiting their overstimulation by glutamate.[181][182] Memantine has been shown to have a small benefit in the treatment of moderate to severe Alzheimer's disease.[183] Reported adverse events with memantine are infrequent and mild, including hallucinations, confusion, dizziness, headache and fatigue.[184] The combination of memantine and donepezil has been shown to be "of statistically significant but clinically marginal effectiveness".[185]
Atypical antipsychotics are modestly useful in reducing aggression and psychosis in people with Alzheimer's disease, but their advantages are offset by serious adverse effects, such as stroke, movement difficulties or cognitive decline.[186] When used in the long-term, they have been shown to associate with increased mortality.[187] Stopping antipsychotic use in this group of people appears to be safe.[188]
Psychosocial intervention
Psychosocial interventions are used as an adjunct to pharmaceutical treatment and can be classified within behaviour-, emotion-, cognition- or stimulation-oriented approaches. Research on efficacy is unavailable and rarely specific to AD, focusing instead on dementia in general.[189]
Behavioural interventions attempt to identify and reduce the antecedents and consequences of problem behaviours. This approach has not shown success in improving overall functioning,[190] but can help to reduce some specific problem behaviours, such as incontinence.[191] There is a lack of high quality data on the effectiveness of these techniques in other behaviour problems such as wandering.[192][193] Music therapy is effective in reducing behavioural and psychological symptoms.[194]
Emotion-oriented interventions include reminiscence therapy, validation therapy, supportive psychotherapy, sensory integration, also called snoezelen, and simulated presence therapy. A Cochrane review has found no evidence that this is effective.[195] Supportive psychotherapy has received little or no formal scientific study, but some clinicians find it useful in helping mildly impaired people adjust to their illness.[189] Reminiscence therapy (RT) involves the discussion of past experiences individually or in group, many times with the aid of photographs, household items, music and sound recordings, or other familiar items from the past. A 2018 review of the effectiveness of RT found that effects were inconsistent, small in size and of doubtful clinical significance, and varied by setting.[196] Simulated presence therapy (SPT) is based on attachment theories and involves playing a recording with voices of the closest relatives of the person with Alzheimer's disease. There is partial evidence indicating that SPT may reduce challenging behaviours.[197] Finally, validation therapy is based on acceptance of the reality and personal truth of another's experience, while sensory integration is based on exercises aimed to stimulate senses. There is no evidence to support the usefulness of these therapies.[198][199]
The aim of cognition-oriented treatments, which include reality orientation and cognitive retraining, is the reduction of cognitive deficits. Reality orientation consists in the presentation of information about time, place or person to ease the understanding of the person about its surroundings and his or her place in them. On the other hand, cognitive retraining tries to improve impaired capacities by exercitation of mental abilities. Both have shown some efficacy improving cognitive capacities,[200] although in some studies these effects were transient and negative effects, such as frustration, have also been reported.[189]
Stimulation-oriented treatments include art, music and pet therapies, exercise, and any other kind of recreational activities. Stimulation has modest support for improving behaviour, mood, and, to a lesser extent, function. Nevertheless, as important as these effects are, the main support for the use of stimulation therapies is the change in the person's routine.[189]
Caregiving
Since Alzheimer's has no cure and it gradually renders people incapable of tending for their own needs, caregiving is essentially the treatment and must be carefully managed over the course of the disease.
During the early and moderate stages, modifications to the living environment and lifestyle can increase patient safety and reduce caretaker burden.[201][202] Examples of such modifications are the adherence to simplified routines, the placing of safety locks, the labelling of household items to cue the person with the disease or the use of modified daily life objects.[189][203][204] If eating becomes problematic, food will need to be prepared in smaller pieces or even pureed.[205] When swallowing difficulties arise, the use of feeding tubes may be required. In such cases, the medical efficacy and ethics of continuing feeding is an important consideration of the caregivers and family members.[206][207] The use of physical restraints is rarely indicated in any stage of the disease, although there are situations when they are necessary to prevent harm to the person with AD or their caregivers.[189]
As the disease progresses, different medical issues can appear, such as oral and dental disease, pressure ulcers, malnutrition, hygiene problems, or respiratory, skin, or eye infections. Careful management can prevent them, while professional treatment is needed when they do arise.[208][209] During the final stages of the disease, treatment is centred on relieving discomfort until death, often with the help of hospice.[210]
Prognosis
The early stages of Alzheimer's disease are difficult to diagnose. A definitive diagnosis is usually made once cognitive impairment compromises daily living activities, although the person may still be living independently. The symptoms will progress from mild cognitive problems, such as memory loss through increasing stages of cognitive and non-cognitive disturbances, eliminating any possibility of independent living, especially in the late stages of the disease.[29]
Life expectancy of people with AD is reduced.[211] Following diagnosis it typically ranges from three to ten years.[211]
Fewer than 3% of people live more than fourteen years.[212] Disease features significantly associated with reduced survival are an increased severity of cognitive impairment, decreased functional level, history of falls, and disturbances in the neurological examination. Other coincident diseases such as heart problems, diabetes or history of alcohol abuse are also related with shortened survival.[213][214][215] While the earlier the age at onset the higher the total survival years, life expectancy is particularly reduced when compared to the healthy population among those who are younger.[216] Men have a less favourable survival prognosis than women.[212][217]
Pneumonia and dehydration are the most frequent immediate causes of death brought by AD, while cancer is a less frequent cause of death than in the general population.[217]
Epidemiology
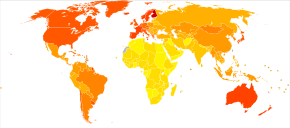
| Age | New affected per thousand person–years |
|---|---|
| 65–69 | 3 |
| 70–74 | 6 |
| 75–79 | 9 |
| 80–84 | 23 |
| 85–89 | 40 |
| 90– | 69 |
Two main measures are used in epidemiological studies: incidence and prevalence. Incidence is the number of new cases per unit of person–time at risk (usually number of new cases per thousand person–years); while prevalence is the total number of cases of the disease in the population at any given time.
Regarding incidence, cohort longitudinal studies (studies where a disease-free population is followed over the years) provide rates between 10 and 15 per thousand person–years for all dementias and 5–8 for AD,[218][219] which means that half of new dementia cases each year are AD. Advancing age is a primary risk factor for the disease and incidence rates are not equal for all ages: every five years after the age of 65, the risk of acquiring the disease approximately doubles, increasing from 3 to as much as 69 per thousand person years.[218][219] There are also sex differences in the incidence rates, women having a higher risk of developing AD particularly in the population older than 85.[219][220] In the United States, the risk of dying from Alzheimer's disease is 26% higher among the non-Hispanic white population than among the non-Hispanic black population, whereas the Hispanic population has a 30% lower risk than the non-Hispanic white population.[221]
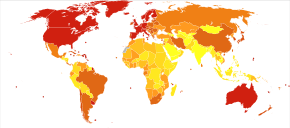
Prevalence of AD in populations is dependent upon different factors including incidence and survival. Since the incidence of AD increases with age, it is particularly important to include the mean age of the population of interest. In the United States, Alzheimer prevalence was estimated to be 1.6% in 2000 both overall and in the 65–74 age group, with the rate increasing to 19% in the 75–84 group and to 42% in the greater than 84 group.[222] Prevalence rates in less developed regions are lower.[223] The World Health Organization estimated that in 2005, 0.379% of people worldwide had dementia, and that the prevalence would increase to 0.441% in 2015 and to 0.556% in 2030.[224] Other studies have reached similar conclusions.[223] Another study estimated that in 2006, 0.40% of the world population (range 0.17–0.89%; absolute number 26.6 million, range 11.4–59.4 million) were afflicted by AD, and that the prevalence rate would triple and the absolute number would quadruple by 2050.[225]
History

The ancient Greek and Roman philosophers and physicians associated old age with increasing dementia.[18] It was not until 1901 that German psychiatrist Alois Alzheimer identified the first case of what became known as Alzheimer's disease, named after him, in a fifty-year-old woman he called Auguste D. He followed her case until she died in 1906, when he first reported publicly on it.[226] During the next five years, eleven similar cases were reported in the medical literature, some of them already using the term Alzheimer's disease.[18] The disease was first described as a distinctive disease by Emil Kraepelin after suppressing some of the clinical (delusions and hallucinations) and pathological features (arteriosclerotic changes) contained in the original report of Auguste D.[227] He included Alzheimer's disease, also named presenile dementia by Kraepelin, as a subtype of senile dementia in the eighth edition of his Textbook of Psychiatry, published on 15 July, 1910.[228]
For most of the 20th century, the diagnosis of Alzheimer's disease was reserved for individuals between the ages of 45 and 65 who developed symptoms of dementia. The terminology changed after 1977 when a conference on AD concluded that the clinical and pathological manifestations of presenile and senile dementia were almost identical, although the authors also added that this did not rule out the possibility that they had different causes.[229] This eventually led to the diagnosis of Alzheimer's disease independent of age.[230] The term senile dementia of the Alzheimer type (SDAT) was used for a time to describe the condition in those over 65, with classical Alzheimer's disease being used to describe those who were younger. Eventually, the term Alzheimer's disease was formally adopted in medical nomenclature to describe individuals of all ages with a characteristic common symptom pattern, disease course, and neuropathology.[231]
Society and culture
Social costs
Dementia, and specifically Alzheimer's disease, may be among the most costly diseases for society in Europe and the United States,[19][20] while their costs in other countries such as Argentina,[232] and South Korea,[233] are also high and rising. These costs will probably increase with the ageing of society, becoming an important social problem. AD-associated costs include direct medical costs such as nursing home care, direct nonmedical costs such as in-home day care, and indirect costs such as lost productivity of both patient and caregiver.[20] Numbers vary between studies but dementia costs worldwide have been calculated around $160 billion,[234] while costs of Alzheimer's disease in the United States may be $100 billion each year.[20]
The greatest origin of costs for society is the long-term care by health care professionals and particularly institutionalisation, which corresponds to 2/3 of the total costs for society.[19] The cost of living at home is also very high,[19] especially when informal costs for the family, such as caregiving time and caregiver's lost earnings, are taken into account.[235]
Costs increase with dementia severity and the presence of behavioural disturbances,[236] and are related to the increased caregiving time required for the provision of physical care.[235] Therefore, any treatment that slows cognitive decline, delays institutionalisation or reduces caregivers' hours will have economic benefits. Economic evaluations of current treatments have shown positive results.[20]
Caregiving burden
The role of the main caregiver is often taken by the spouse or a close relative.[237] Alzheimer's disease is known for placing a great burden on caregivers which includes social, psychological, physical or economic aspects.[14][238][239] Home care is usually preferred by people with AD and their families.[240] This option also delays or eliminates the need for more professional and costly levels of care.[240][241] Nevertheless, two-thirds of nursing home residents have dementias.[189]
Dementia caregivers are subject to high rates of physical and mental disorders.[242] Factors associated with greater psychosocial problems of the primary caregivers include having an affected person at home, the carer being a spouse, demanding behaviours of the cared person such as depression, behavioural disturbances, hallucinations, sleep problems or walking disruptions and social isolation.[243][244] Regarding economic problems, family caregivers often give up time from work to spend 47 hours per week on average with the person with AD, while the costs of caring for them are high. Direct and indirect costs of caring for an Alzheimer's patient average between $18,000 and $77,500 per year in the United States, depending on the study.[235][237]
Cognitive behavioural therapy and the teaching of coping strategies either individually or in group have demonstrated their efficacy in improving caregivers' psychological health.[14][245]
Media
AD has been portrayed in films such as: Iris (2001), based on John Bayley's memoir of his wife Iris Murdoch;[246] The Notebook (2004), based on Nicholas Sparks' 1996 novel of the same name;[247] A Moment to Remember (2004); Thanmathra (2005);[248] Memories of Tomorrow (Ashita no Kioku) (2006), based on Hiroshi Ogiwara's novel of the same name;[249] Away from Her (2006), based on Alice Munro's short story "The Bear Came over the Mountain";[250] Still Alice (2014), about a Columbia University professor who has early onset Alzheimer's disease, based on Lisa Genova's 2007 novel of the same name and featuring Julianne Moore in the title role. Documentaries on Alzheimer's disease include Malcolm and Barbara: A Love Story (1999) and Malcolm and Barbara: Love's Farewell (2007), both featuring Malcolm Pointon.[251][252][253]
Research directions
Medication
In the decade 2002–2012, 244 compounds were assessed in Phase I, Phase II, or Phase III trials, and only one of these (memantine) received FDA approval (though others were still in the pipeline).[254] Solanezumab and aducanumab failed to show effectiveness in people who already had Alzheimer's symptoms.[255]
One area of clinical research is focused on treating the underlying disease pathology. Reduction of beta-amyloid levels is a common target of compounds[256] (such as apomorphine) under investigation. Immunotherapy or vaccination for the amyloid protein is one treatment modality under study.[257] Unlike preventive vaccination, the putative therapy would be used to treat people already diagnosed. It is based upon the concept of training the immune system to recognise, attack, and reverse deposition of amyloid, thereby altering the course of the disease.[258] An example of such a vaccine under investigation was ACC-001,[259][260] although the trials were suspended in 2008.[261] Another similar agent is bapineuzumab, an antibody designed as identical to the naturally induced anti-amyloid antibody.[262] However, immunotherapeutic agents have been found to cause some concerning adverse drug reactions, such as amyloid-related imaging abnormalities.[263] Other approaches are neuroprotective agents, such as AL-108,[264] and metal-protein interaction attenuation agents, such as PBT2.[265] A TNFα receptor-blocking fusion protein, etanercept has showed encouraging results.[266]
In 2008, two separate clinical trials showed positive results in modifying the course of disease in mild to moderate AD with methylthioninium chloride, a drug that inhibits tau aggregation,[267][268] and dimebon, an antihistamine.[269] The consecutive phase-III trial of dimebon failed to show positive effects in the primary and secondary endpoints.[270][271][272] Work with methylthioninium chloride showed that bioavailability of methylthioninium from the gut was affected by feeding and by stomach acidity, leading to unexpectedly variable dosing.[273] A new stabilised formulation, as the prodrug LMTX, is in phase-III trials (in 2014).[274]
In early 2017, a trial of verubecestat, which inhibits the beta-secretase protein responsible for creating beta-amyloid protein was discontinued as an independent panel found "virtually no chance of finding a positive clinical effect".[275] In 2018 and 2019, more trials, including aducanumab which reduced amyloid beta concentrations, failed, leading some to question the validity of the amyloid hypothesis.[276][277] However, in October 2019, an analysis of another dataset found that aducanumab may reduce clinical decline in people with early Alzheimer's disease and the Biogen company may seek regulatory approval again.[278]
The senescence accelerated mouse (SAMP8) is an Alzheimer's disease (AD) animal model in which amyloid precursor protein (APP) is overproduced. The mice develops early memory disturbances and alters the blood–brain barrier, which causes a decreased expulsion of amyloid-β protein from the brain. It has a marked increase in oxidative stress in the brain. Medications that reduce oxidative stress have been shown to improve memory. Treatments that reduce amyloid-β (antisense to APP and antibodies to amyloid-β) not only improve memory but also reduce oxidative stress. It has been shown that the initial deviations in lipid peroxidative damage favor mitochondrial dysfunction as being a trigger for amyloid-β overproduction in this AD mouse strain. This process begets increased amyloid-beta, which further damages mitochondria.[279]
Behavioral prevention
Research on the effects of meditation on preserving memory and cognitive functions is at an early stage.[280] A 2015 review suggests that mindfulness-based interventions may prevent or delay the onset of mild cognitive impairment and Alzheimer's disease.[281]
Possible transmission
Rare cases of possible transmission between people are being studied,[282] e.g. to growth hormone patients.[283]
Infections
The herpes simplex virus HSV-1 has been found in the same areas as amyloid plaques.[284] This suggested the possibility that AD could be treated or prevented with antiviral medication.[284][285] Studies of antivirals in cell cultures have shown promising results.[286]
Fungal infection of AD brain has also been described.[287] This hypothesis was proposed by the microbiologist L. Carrasco when his group found statistical correlation between disseminated mycoses and AD.[288] Further work revealed that fungal infection is present in different brain regions of AD patients, but not in the control individuals.[289] [290] A fungal infection explains the symptoms observed in AD patients. The slow progression of AD fits with the chronic nature of some systemic fungal infections, which can be asymptomatic and thus, unnoticed and untreated.[289] The fungal hypotheses are also compatible with some other established AD hypotheses, like the amyloid hypothesis, that can be explained as an immune system response to an infection in the CNS,[291][292][293] as found by R. Moir and R. Tanzi in mouse and worm models of AD.
Imaging
Of the many medical imaging techniques available, single photon emission computed tomography (SPECT) appears to be superior in differentiating Alzheimer's disease from other types of dementia, and this has been shown to give a greater level of accuracy compared with mental testing and medical history analysis.[294] Advances have led to the proposal of new diagnostic criteria.[22][121]
PiB PET remains investigational, but a similar PET scanning radiopharmaceutical called florbetapir, containing the longer-lasting radionuclide fluorine-18, is a diagnostic tool in Alzheimer's disease.[295][296]
Amyloid imaging is likely to be used in conjunction with other markers rather than as an alternative.[297] Volumetric MRI can detect changes in the size of brain regions. Measuring those regions that atrophy during the progress of Alzheimer's disease is showing promise as a diagnostic indicator. It may prove less expensive than other imaging methods currently under study.[298]
In 2011, an FDA panel voted unanimously to recommend approval of florbetapir.[299] The imaging agent can help to detect Alzheimer's brain plaques.[300] A negative scan indicates sparse or no plaques, which is not consistent with a diagnosis of AD.[301]
Diagnosis
Emphasis in Alzheimer's research has been placed on diagnosing the condition before symptoms begin.[302] A number of biochemical tests have been developed to enable earlier detection. Some such tests involve the analysis of cerebrospinal fluid for beta-amyloid, total tau protein and phosphorylated tau181P protein concentrations.[303] Because drawing CSF can be painful, repeated draws are avoided. A blood test for circulatory miRNA and inflammatory biomarkers is a potential alternative indicator.[303]
A series of studies suggest that ageing-related breakdown of the blood–brain barrier may be causative of AD, and conclude that markers for that damage may be an early predictor of the disease.[304][305][306]
References
- Burns A, Iliffe S (February 2009). "Alzheimer's disease". BMJ. 338: b158. doi:10.1136/bmj.b158. PMID 19196745. S2CID 8570146.
- "Dementia Fact sheet". World Health Organization. 12 December 2017.
- Mendez MF (November 2012). "Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD". Archives of Medical Research. 43 (8): 677–85. doi:10.1016/j.arcmed.2012.11.009. PMC 3532551. PMID 23178565.
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (March 2011). "Alzheimer's disease". Lancet. 377 (9770): 1019–31. doi:10.1016/S0140-6736(10)61349-9. PMID 21371747.
- "Dementia diagnosis and assessment" (PDF). National Institute for Health and Care Excellence (NICE). Archived from the original (PDF) on 5 December 2014. Retrieved 30 November 2014.
- Commission de la transparence (June 2012). "Drugs for Alzheimer's disease: best avoided. No therapeutic advantage" [Drugs for Alzheimer's disease: best avoided. No therapeutic advantage]. Prescrire International. 21 (128): 150. PMID 22822592.
- Querfurth HW, LaFerla FM (January 2010). "Alzheimer's disease". The New England Journal of Medicine. 362 (4): 329–44. doi:10.1056/NEJMra0909142. PMID 20107219. S2CID 205115756.
- GBD 2015 Disease Injury Incidence Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- GBD 2015 Mortality Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- "About Alzheimer's Disease: Symptoms". National Institute on Aging. Archived from the original on 15 January 2012. Retrieved 28 December 2011.
- Todd S, Barr S, Roberts M, Passmore AP (November 2013). "Survival in dementia and predictors of mortality: a review". International Journal of Geriatric Psychiatry. 28 (11): 1109–24. doi:10.1002/gps.3946. PMID 23526458.
- "So, What Can You Do?". National Institute on Aging. 29 July 2016. Archived from the original on 3 April 2017.
- Hsu D, Marshall GA (2017). "Primary and Secondary Prevention Trials in Alzheimer Disease: Looking Back, Moving Forward". Current Alzheimer Research. 14 (4): 426–40. doi:10.2174/1567205013666160930112125. PMC 5329133. PMID 27697063.
- Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J (July 2007). "Systematic review of information and support interventions for caregivers of people with dementia". BMC Geriatrics. 7: 18. doi:10.1186/1471-2318-7-18. PMC 1951962. PMID 17662119.
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S (April 2015). "Exercise programs for people with dementia". The Cochrane Database of Systematic Reviews (Submitted manuscript). 132 (4): CD006489. doi:10.1002/14651858.CD006489.pub4. PMID 25874613.
- National Institute for Health and Clinical Excellence. "Low-dose antipsychotics in people with dementia". National Institute for Health and Care Excellence (NICE). Archived from the original on 5 December 2014. Retrieved 29 November 2014.
- "Information for Healthcare Professionals: Conventional Antipsychotics". US Food and Drug Administration. 16 June 2008. Archived from the original on 29 November 2014. Retrieved 29 November 2014.
- Berchtold NC, Cotman CW (1998). "Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s". Neurobiology of Aging. 19 (3): 173–89. doi:10.1016/S0197-4580(98)00052-9. PMID 9661992.
- Bonin-Guillaume S, Zekry D, Giacobini E, Gold G, Michel JP (January 2005). "[The economical impact of dementia]". Presse Médicale (in French). 34 (1): 35–41. doi:10.1016/s0755-4982(05)83882-5. PMID 15685097.
- Meek PD, McKeithan K, Schumock GT (1998). "Economic considerations in Alzheimer's disease". Pharmacotherapy. 18 (2 Pt 2): 68–73, discussion 79–82. doi:10.1002/j.1875-9114.1998.tb03880.x (inactive 25 May 2020). PMID 9543467.
- "Evaluating Prescription Drugs Used to Treat: Alzheimer's Disease Comparing Effectiveness, Safety, and Price" (PDF). Consumer Reports Drug Effectiveness Review Project. Consumer Reports. May 2012. Archived from the original (PDF) on 5 September 2012. Retrieved 1 May 2013.
- Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B (January 2007). "Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline". European Journal of Neurology. 14 (1): e1–26. doi:10.1111/j.1468-1331.2006.01605.x. PMID 17222085.
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ (September 2004). "Multiple cognitive deficits during the transition to Alzheimer's disease". Journal of Internal Medicine. 256 (3): 195–204. doi:10.1111/j.1365-2796.2004.01386.x. PMID 15324363.
- Nygård L (2003). "Instrumental activities of daily living: a stepping-stone towards Alzheimer's disease diagnosis in subjects with mild cognitive impairment?". Acta Neurologica Scandinavica. Supplementum. 179 (s179): 42–6. doi:10.1034/j.1600-0404.107.s179.8.x. PMID 12603250.
- Arnáiz E, Almkvist O (2003). "Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease". Acta Neurologica Scandinavica. Supplementum. 179: 34–41. doi:10.1034/j.1600-0404.107.s179.7.x. PMID 12603249.
- Landes AM, Sperry SD, Strauss ME, Geldmacher DS (December 2001). "Apathy in Alzheimer's disease". Journal of the American Geriatrics Society. 49 (12): 1700–7. doi:10.1046/j.1532-5415.2001.49282.x. PMID 11844006.
- Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's neurology in clinical practice (6th ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-0434-1.
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. (January 2004). "Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials". Archives of Neurology. 61 (1): 59–66. doi:10.1001/archneur.61.1.59. PMID 14732621.
- Förstl H, Kurz A (1999). "Clinical features of Alzheimer's disease". European Archives of Psychiatry and Clinical Neuroscience. 249 (6): 288–90. doi:10.1007/s004060050101. PMID 10653284.
- Carlesimo GA, Oscar-Berman M (June 1992). "Memory deficits in Alzheimer's patients: a comprehensive review". Neuropsychology Review. 3 (2): 119–69. doi:10.1007/BF01108841. PMID 1300219.
- Jelicic M, Bonebakker AE, Bonke B (1995). "Implicit memory performance of patients with Alzheimer's disease: a brief review". International Psychogeriatrics. 7 (3): 385–92. doi:10.1017/S1041610295002134. PMID 8821346.
- Taler V, Phillips NA (July 2008). "Language performance in Alzheimer's disease and mild cognitive impairment: a comparative review". Journal of Clinical and Experimental Neuropsychology. 30 (5): 501–56. doi:10.1080/13803390701550128. PMID 18569251.
- Frank EM (September 1994). "Effect of Alzheimer's disease on communication function". Journal of the South Carolina Medical Association. 90 (9): 417–23. PMID 7967534.
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A (May 2001). "Sundowning and circadian rhythms in Alzheimer's disease". The American Journal of Psychiatry. 158 (5): 704–11. doi:10.1176/appi.ajp.158.5.704. PMID 11329390. S2CID 10492607.
- Gold DP, Reis MF, Markiewicz D, Andres D (January 1995). "When home caregiving ends: a longitudinal study of outcomes for caregivers of relatives with dementia". Journal of the American Geriatrics Society. 43 (1): 10–6. doi:10.1111/j.1532-5415.1995.tb06235.x. PMID 7806732.
- "What We Know Today About Alzheimer's Disease". Alzheimer's Association. Archived from the original on 7 October 2011. Retrieved 1 October 2011.
While scientists know Alzheimer's disease involves progressive brain cell failure, the reason cells fail isn't clear.
- Reitz C, Mayeux R (April 2014). "Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers". Biochemical Pharmacology. 88 (4): 640–51. doi:10.1016/j.bcp.2013.12.024. PMC 3992261. PMID 24398425.
- Wilson RS, Barral S, Lee JH, Leurgans SE, Foroud TM, Sweet RA, Graff-Radford N, Bird TD, Mayeux R, Bennett DA (2011). "Heritability of different forms of memory in the Late Onset Alzheimer's Disease Family Study". Journal of Alzheimer's Disease. 23 (2): 249–55. doi:10.3233/JAD-2010-101515. PMC 3130303. PMID 20930268.
- Blennow K, de Leon MJ, Zetterberg H (July 2006). "Alzheimer's disease". Lancet. 368 (9533): 387–403. doi:10.1016/S0140-6736(06)69113-7. PMID 16876668.
- Waring SC, Rosenberg RN (March 2008). "Genome-wide association studies in Alzheimer disease". Archives of Neurology. 65 (3): 329–34. doi:10.1001/archneur.65.3.329. PMID 18332245.
- Selkoe DJ (June 1999). "Translating cell biology into therapeutic advances in Alzheimer's disease". Nature. 399 (6738 Suppl): A23–31. doi:10.1038/19866. PMID 10392577.
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, et al. (November 1996). "Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo". Neuron. 17 (5): 1005–13. doi:10.1016/S0896-6273(00)80230-5. PMID 8938131.
- Kim, JH (December 2018). "Genetics of Alzheimer's Disease". Dementia and Neurocognitive Disorders. 17 (4): 131–36. doi:10.12779/dnd.2018.17.4.131. PMC 6425887. PMID 30906402.
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD (March 1993). "Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease". Proceedings of the National Academy of Sciences of the United States of America. 90 (5): 1977–81. Bibcode:1993PNAS...90.1977S. doi:10.1073/pnas.90.5.1977. PMC 46003. PMID 8446617.
- Mahley RW, Weisgraber KH, Huang Y (April 2006). "Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease". Proceedings of the National Academy of Sciences of the United States of America. 103 (15): 5644–51. Bibcode:2006PNAS..103.5644M. doi:10.1073/pnas.0600549103. PMC 1414631. PMID 16567625.
- Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H (January 2006). "Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba". Neurology. 66 (2): 223–27. doi:10.1212/01.wnl.0000194507.39504.17. PMC 2860622. PMID 16434658.
- Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, et al. (January 2006). "APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians". Annals of Neurology. 59 (1): 182–85. doi:10.1002/ana.20694. PMC 2855121. PMID 16278853.
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. (December 2013). "Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease". Nature Genetics. 45 (12): 1452–58. doi:10.1038/ng.2802. PMC 3896259. PMID 24162737.
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. (January 2013). "Variant of TREM2 associated with the risk of Alzheimer's disease". The New England Journal of Medicine (Original article). 368 (2): 107–16. doi:10.1056/NEJMoa1211103. PMC 3677583. PMID 23150908.
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. (January 2013). "TREM2 variants in Alzheimer's disease". The New England Journal of Medicine (Original article). 368 (2): 117–27. doi:10.1056/NEJMoa1211851. PMC 3631573. PMID 23150934.
- Mukherjee S, Mez J, Trittschuh EH, Saykin AJ, Gibbons LE, Fardo DW, et al. (December 2018). "Genetic data and cognitively defined late-onset Alzheimer's disease subgroups". Molecular Psychiatry. doi:10.1038/s41380-018-0298-8. PMC 6548676. PMID 30514930.
- Francis PT, Palmer AM, Snape M, Wilcock GK (February 1999). "The cholinergic hypothesis of Alzheimer's disease: a review of progress". Journal of Neurology, Neurosurgery, and Psychiatry. 66 (2): 137–47. doi:10.1136/jnnp.66.2.137. PMC 1736202. PMID 10071091.
- Martorana A, Esposito Z, Koch G (August 2010). "Beyond the cholinergic hypothesis: do current drugs work in Alzheimer's disease?". CNS Neuroscience & Therapeutics. 16 (4): 235–45. doi:10.1111/j.1755-5949.2010.00175.x. PMC 6493875. PMID 20560995.
- Hardy J, Allsop D (October 1991). "Amyloid deposition as the central event in the aetiology of Alzheimer's disease". Trends in Pharmacological Sciences. 12 (10): 383–88. doi:10.1016/0165-6147(91)90609-V. PMID 1763432.
- Mudher A, Lovestone S (January 2002). "Alzheimer's disease-do tauists and baptists finally shake hands?". Trends in Neurosciences. 25 (1): 22–26. doi:10.1016/S0166-2236(00)02031-2. PMID 11801334.
- Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E (October 2007). "Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain". Neurobiology of Aging. 28 (10): 1493–506. doi:10.1016/j.neurobiolaging.2006.06.023. PMC 3375834. PMID 16904243.
- Lott IT, Head E (March 2005). "Alzheimer disease and Down syndrome: factors in pathogenesis". Neurobiology of Aging. 26 (3): 383–89. doi:10.1016/j.neurobiolaging.2004.08.005. PMID 15639317.
- Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinistö L, Halonen P, Kontula K (November 1995). "Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein". The New England Journal of Medicine. 333 (19): 1242–47. doi:10.1056/NEJM199511093331902. PMID 7566000.
- Transgenic mice:
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F (February 1995). "Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein". Nature. 373 (6514): 523–27. Bibcode:1995Natur.373..523G. doi:10.1038/373523a0. PMID 7845465.
- Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D (September 1996). "Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease". The Journal of Neuroscience. 16 (18): 5795–811. doi:10.1523/JNEUROSCI.16-18-05795.1996. PMC 6578961. PMID 8795633.
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (October 1996). "Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice". Science. 274 (5284): 99–102. Bibcode:1996Sci...274...99H. doi:10.1126/science.274.5284.99. PMID 8810256.
- Lalonde R, Dumont M, Staufenbiel M, Sturchler-Pierrat C, Strazielle C (November 2002). "Spatial learning, exploration, anxiety, and motor coordination in female APP23 transgenic mice with the Swedish mutation". Brain Research. 956 (1): 36–44. doi:10.1016/S0006-8993(02)03476-5. PMID 12426044.
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA (July 2008). "Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial". Lancet. 372 (9634): 216–23. doi:10.1016/S0140-6736(08)61075-2. PMID 18640458.
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. (January 2007). "Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease". The Journal of Neuroscience. 27 (4): 796–807. doi:10.1523/JNEUROSCI.3501-06.2007. PMC 6672917. PMID 17251419.
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (February 2009). "Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers". Nature. 457 (7233): 1128–32. Bibcode:2009Natur.457.1128L. doi:10.1038/nature07761. PMC 2748841. PMID 19242475.
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M (February 2009). "APP binds DR6 to trigger axon pruning and neuron death via distinct caspases". Nature. 457 (7232): 981–89. Bibcode:2009Natur.457..981N. doi:10.1038/nature07767. PMC 2677572. PMID 19225519.
- Tomiyama T (2010). "Involvement of beta-amyloid in the etiology of Alzheimer's disease". Brain Nerve. 62 (7): 691–699. PMID 20675873.
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H (2008). "A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia". Ann Neurol. 63 (3): 377–387. doi:10.1002/ana.21321. PMID 18300294.CS1 maint: multiple names: authors list (link)
- Goedert M, Spillantini MG, Crowther RA (July 1991). "Tau proteins and neurofibrillary degeneration". Brain Pathology. 1 (4): 279–86. doi:10.1111/j.1750-3639.1991.tb00671.x. PMID 1669718.
- Iqbal K, Alonso A, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I (January 2005). "Tau pathology in Alzheimer disease and other tauopathies". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1739 (2–3): 198–210. doi:10.1016/j.bbadis.2004.09.008. PMID 15615638.
- Chun W, Johnson GV (January 2007). "The role of tau phosphorylation and cleavage in neuronal cell death". Frontiers in Bioscience. 12: 733–56. doi:10.2741/2097. PMID 17127334. S2CID 40048768.
- Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ (July 2008). "Inflammation and Alzheimer's disease: possible role of periodontal diseases". Alzheimer's & Dementia. 4 (4): 242–50. doi:10.1016/j.jalz.2007.08.004. PMID 18631974.
- Collins SM, Surette M, Bercik P (November 2012). "The interplay between the intestinal microbiota and the brain". Nature Reviews. Microbiology. 10 (11): 735–42. doi:10.1038/nrmicro2876. PMID 23000955.
- Deane R, Zlokovic BV (April 2007). "Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease". Current Alzheimer Research. 4 (2): 191–97. doi:10.2174/156720507780362245. PMID 17430246.
- Miklossy J (August 2011). "Alzheimer's disease - a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria". Journal of Neuroinflammation. 8 (1): 90. doi:10.1186/1742-2094-8-90. PMC 3171359. PMID 21816039.
- Allen, HB (27 June 2016). "Alzheimer's Disease: Assessing the Role of Spirochetes, Biofilms, the Immune System, and Amyloid-β with Regard to Potential Treatment and Prevention". Journal of Alzheimer's Disease. 53 (4): 1271–76. doi:10.3233/JAD-160388. PMC 5008232. PMID 27372648.
- Xu H, Finkelstein DI, Adlard PA (12 June 2014). "Interactions of metals and Apolipoprotein E in Alzheimer's disease". Frontiers in Aging Neuroscience. 6: 121. doi:10.3389/fnagi.2014.00121. PMC 4054654. PMID 24971061.
Although we still do not know if the metal ion dyshomeostasis present in AD is a cause or consequence of the disease, there is a growing body of evidence showing a direct correlation between metal ions and key AD-related key proteins.
- Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X (December 2008). "Oxidative stress signaling in Alzheimer's disease". Current Alzheimer Research. 5 (6): 525–32. doi:10.2174/156720508786898451. PMC 2780015. PMID 19075578.
- Kastenholz B, Garfin DE, Horst J, Nagel KA (2009). "Plant metal chaperones: a novel perspective in dementia therapy". Amyloid. 16 (2): 81–83. doi:10.1080/13506120902879392. PMID 20536399.
- "Aluminium and Alzheimer's disease". Facts about dementia. Alzheimer's Society. Archived from the original on 27 October 2005. Retrieved 14 October 2005.
- Bondy SC (January 2016). "Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer's disease and age-related neurodegeneration". Neurotoxicology (Submitted manuscript). 52: 222–29. doi:10.1016/j.neuro.2015.12.002. PMID 26687397.
- Kandimalla R, Vallamkondu J, Corgiat EB, Gill KD (March 2016). "Understanding Aspects of Aluminum Exposure in Alzheimer's Disease Development". Brain Pathology. 26 (2): 139–54. doi:10.1111/bpa.12333. PMID 26494454.
- Santibáñez M, Bolumar F, García AM (November 2007). "Occupational risk factors in Alzheimer's disease: a review assessing the quality of published epidemiological studies". Occupational and Environmental Medicine. 64 (11): 723–32. doi:10.1136/oem.2006.028209. PMC 2078415. PMID 17525096.
- Lidsky TI (May 2014). "Is the Aluminum Hypothesis dead?". Journal of Occupational and Environmental Medicine. 56 (5 Suppl): S73–79. doi:10.1097/jom.0000000000000063. PMC 4131942. PMID 24806729.
- Yegambaram M, Manivannan B, Beach TG, Halden RU (2015). "Role of environmental contaminants in the etiology of Alzheimer's disease: a review". Current Alzheimer Research. 12 (2): 116–46. doi:10.2174/1567205012666150204121719. PMC 4428475. PMID 25654508.
- Cataldo JK, Prochaska JJ, Glantz SA (2010). "Cigarette smoking is a risk factor for Alzheimer's Disease: an analysis controlling for tobacco industry affiliation". Journal of Alzheimer's Disease. 19 (2): 465–80. doi:10.3233/JAD-2010-1240. PMC 2906761. PMID 20110594.
- Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA (2010). "Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer's disease". Neuro-Degenerative Diseases. 7 (1–3): 38–41. doi:10.1159/000283480. PMID 20160456.
- Moulton PV, Yang W (2012). "Air pollution, oxidative stress, and Alzheimer's disease". Journal of Environmental and Public Health (Review). 2012: 1–9. doi:10.1155/2012/472751. PMC 3317180. PMID 22523504.
- Bartzokis G (August 2011). "Alzheimer's disease as homeostatic responses to age-related myelin breakdown". Neurobiology of Aging. 32 (8): 1341–71. doi:10.1016/j.neurobiolaging.2009.08.007. PMC 3128664. PMID 19775776.
- Cai Z, Xiao M (2016). "Oligodendrocytes and Alzheimer's disease". The International Journal of Neuroscience. 126 (2): 97–104. doi:10.3109/00207454.2015.1025778. PMID 26000818.
- Reisberg B, Franssen EH, Hasan SM, Monteiro I, Boksay I, Souren LE, et al. (1999). "Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer's and other dementing processes". European Archives of Psychiatry and Clinical Neuroscience. 249 Suppl 3 (3): 28–36. doi:10.1007/pl00014170. PMID 10654097.
- Alves GS, Oertel Knöchel V, Knöchel C, Carvalho AF, Pantel J, Engelhardt E, Laks J (2015). "Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity". BioMed Research International. 2015: 291658. doi:10.1155/2015/291658. PMC 4320890. PMID 25685779.
- Brenner Carson, Verna (2015). Caregiving for Alzheimer's Disease. New York: Springer New York Academy of Sciences. pp. 1–9. ISBN 978-1-4939-2406-6.
- Zis P, Hadjivassiliou M (February 2019). "Treatment of Neurological Manifestations of Gluten Sensitivity and Coeliac Disease". Current Treatment Options in Neurology. 21 (3): 10. doi:10.1007/s11940-019-0552-7. PMID 30806821.
- Makhlouf S, Messelmani M, Zaouali J, Mrissa R (March 2018). "Cognitive impairment in celiac disease and non-celiac gluten sensitivity: review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet". Acta Neurologica Belgica (Review). 118 (1): 21–27. doi:10.1007/s13760-017-0870-z. PMID 29247390.
- Wenk GL (2003). "Neuropathologic changes in Alzheimer's disease". The Journal of Clinical Psychiatry. 64 Suppl 9: 7–10. PMID 12934968.
- Braak H, Del Tredici K (December 2012). "Where, when, and in what form does sporadic Alzheimer's disease begin?". Current Opinion in Neurology. 25 (6): 708–14. doi:10.1097/WCO.0b013e32835a3432. PMID 23160422.
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Schmansky NJ, Greve DN, Salat DH, Buckner RL, Fischl B (August 2009). "Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease". Brain. 132 (Pt 8): 2048–57. doi:10.1093/brain/awp123. PMC 2714061. PMID 19460794.
- Moan R (20 July 2009). "MRI Software Accurately IDs Preclinical Alzheimer's Disease". Diagnostic Imaging. Archived from the original on 16 May 2016. Retrieved 7 January 2013.
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (June 2004). "The importance of neuritic plaques and tangles to the development and evolution of AD". Neurology. 62 (11): 1984–89. doi:10.1212/01.WNL.0000129697.01779.0A. PMID 15184601.
- DeTure, Michael A.; Dickson, Dennis W. (2019). "The neuropathological diagnosis of Alzheimer's disease". Molecular Neurodegeneration. 14 (1). doi:10.1186/s13024-019-0333-5. ISSN 1750-1326.
- Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994). "Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital" (PDF). Cerebral Cortex. 4 (2): 138–50. doi:10.1093/cercor/4.2.138. PMID 8038565.
- Kotzbauer PT, Trojanowsk JQ, Lee VM (October 2001). "Lewy body pathology in Alzheimer's disease". Journal of Molecular Neuroscience. 17 (2): 225–32. doi:10.1385/JMN:17:2:225. PMID 11816795.
- Hashimoto M, Rockenstein E, Crews L, Masliah E (2003). "Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases". Neuromolecular Medicine. 4 (1–2): 21–36. doi:10.1385/NMM:4:1-2:21. PMID 14528050.
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (July 2006). "Synapse formation and function is modulated by the amyloid precursor protein". The Journal of Neuroscience. 26 (27): 7212–21. doi:10.1523/JNEUROSCI.1450-06.2006. PMC 6673945. PMID 16822978.
- Turner PR, O'Connor K, Tate WP, Abraham WC (May 2003). "Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory". Progress in Neurobiology. 70 (1): 1–32. doi:10.1016/S0301-0082(03)00089-3. PMID 12927332.
- Hooper NM (April 2005). "Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein". Biochemical Society Transactions. 33 (Pt 2): 335–38. doi:10.1042/BST0330335. PMID 15787600. S2CID 14269634.
- Ohnishi S, Takano K (March 2004). "Amyloid fibrils from the viewpoint of protein folding". Cellular and Molecular Life Sciences. 61 (5): 511–24. doi:10.1007/s00018-003-3264-8. PMID 15004691.
- Hernández F, Avila J (September 2007). "Tauopathies". Cellular and Molecular Life Sciences. 64 (17): 2219–33. doi:10.1007/s00018-007-7220-x. PMID 17604998.
- Sun W, Samimi H, Gamez M, Zare H, Frost B (August 2018). "Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies". Nature Neuroscience. 21 (8): 1038–48. doi:10.1038/s41593-018-0194-1. PMC 6095477. PMID 30038280.
- Van Broeck B, Van Broeckhoven C, Kumar-Singh S (2007). "Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches". Neuro-Degenerative Diseases. 4 (5): 349–65. doi:10.1159/000105156. PMID 17622778.
- Huang Y, Mucke L (March 2012). "Alzheimer mechanisms and therapeutic strategies". Cell. 148 (6): 1204–22. doi:10.1016/j.cell.2012.02.040. PMC 3319071. PMID 22424230.
- Yankner BA, Duffy LK, Kirschner DA (October 1990). "Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides". Science. 250 (4978): 279–82. Bibcode:1990Sci...250..279Y. doi:10.1126/science.2218531. PMID 2218531.
- Chen X, Yan SD (December 2006). "Mitochondrial Abeta: a potential cause of metabolic dysfunction in Alzheimer's disease". IUBMB Life. 58 (12): 686–94. doi:10.1080/15216540601047767. PMID 17424907.
- Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK (December 2004). "New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists". Annals of the New York Academy of Sciences. 1035: 290–315. doi:10.1196/annals.1332.018. PMID 15681814.
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. (April 2015). "Neuroinflammation in Alzheimer's disease". The Lancet. Neurology. 14 (4): 388–405. doi:10.1016/S1474-4422(15)70016-5. PMC 5909703. PMID 25792098.
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (November 2008). "New insights into brain BDNF function in normal aging and Alzheimer disease". Brain Research Reviews. 59 (1): 201–20. doi:10.1016/j.brainresrev.2008.07.007. PMID 18708092.
- Schindowski K, Belarbi K, Buée L (February 2008). "Neurotrophic factors in Alzheimer's disease: role of axonal transport". Genes, Brain, and Behavior. 7 (Suppl 1): 43–56. doi:10.1111/j.1601-183X.2007.00378.x. PMC 2228393. PMID 18184369.
- Mendez MF (2006). "The accurate diagnosis of early-onset dementia". International Journal of Psychiatry in Medicine. 36 (4): 401–12. doi:10.2190/Q6J4-R143-P630-KW41. PMID 17407994.
- Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J (November 2006). "Therapeutic approaches to Alzheimer's disease". Brain. 129 (Pt 11): 2840–55. doi:10.1093/brain/awl280. PMID 17018549.
- Dementia: Quick Reference Guide (PDF). London: (UK) National Institute for Health and Clinical Excellence. November 2006. ISBN 978-1-84629-312-2. Archived from the original (PDF) on 27 February 2008. Retrieved 22 February 2008.
- Schroeter ML, Stein T, Maslowski N, Neumann J (October 2009). "Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients". NeuroImage. 47 (4): 1196–206. doi:10.1016/j.neuroimage.2009.05.037. PMC 2730171. PMID 19463961.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (July 1984). "Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease". Neurology. 34 (7): 939–44. doi:10.1212/wnl.34.7.939. PMID 6610841.
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. (August 2007). "Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria". The Lancet. Neurology. 6 (8): 734–46. doi:10.1016/S1474-4422(07)70178-3. PMID 17616482.
- Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF (December 1994). "Reliability and validity of NINCDS-ADRDA criteria for Alzheimer's disease. The National Institute of Mental Health Genetics Initiative". Archives of Neurology. 51 (12): 1198–204. doi:10.1001/archneur.1994.00540240042014. PMID 7986174.
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th Edition Text Revision ed.). Washington, DC: American Psychiatric Association. ISBN 978-0-89042-025-6.
- Ito N (May 1996). "[Clinical aspects of dementia]". [Hokkaido Igaku Zasshi] the Hokkaido Journal of Medical Science (in Japanese). 71 (3): 315–20. PMID 8752526.
- Tombaugh TN, McIntyre NJ (September 1992). "The mini-mental state examination: a comprehensive review". Journal of the American Geriatrics Society. 40 (9): 922–35. doi:10.1111/j.1532-5415.1992.tb01992.x. PMID 1512391.
- Pasquier F (January 1999). "Early diagnosis of dementia: neuropsychology". Journal of Neurology. 246 (1): 6–15. doi:10.1007/s004150050299. PMID 9987708.
- Harvey PD, Moriarty PJ, Kleinman L, Coyne K, Sadowsky CH, Chen M, Mirski DF (2005). "The validation of a caregiver assessment of dementia: the Dementia Severity Scale". Alzheimer Disease and Associated Disorders. 19 (4): 186–94. doi:10.1097/01.wad.0000189034.43203.60. PMID 16327345.
- Antoine C, Antoine P, Guermonprez P, Frigard B (2004). "[Awareness of deficits and anosognosia in Alzheimer's disease]". L'Encephale (in French). 30 (6): 570–77. doi:10.1016/S0013-7006(04)95472-3. PMID 15738860.
- Cruz VT, Pais J, Teixeira A, Nunes B (2004). "[The initial symptoms of Alzheimer disease: caregiver perception]". Acta Medica Portuguesa (in Portuguese). 17 (6): 435–44. PMID 16197855.
- Clarfield AM (October 2003). "The decreasing prevalence of reversible dementias: an updated meta-analysis". Archives of Internal Medicine. 163 (18): 2219–29. doi:10.1001/archinte.163.18.2219. PMID 14557220.
- Sun X, Steffens DC, Au R, Folstein M, Summergrad P, Yee J, Rosenberg I, Mwamburi DM, Qiu WQ (May 2008). "Amyloid-associated depression: a prodromal depression of Alzheimer disease?". Archives of General Psychiatry. 65 (5): 542–50. doi:10.1001/archpsyc.65.5.542. PMC 3042807. PMID 18458206.
- Geldmacher DS, Whitehouse PJ (May 1997). "Differential diagnosis of Alzheimer's disease". Neurology. 48 (5 Suppl 6): S2–9. doi:10.1212/WNL.48.5_Suppl_6.2S. PMID 9153154.
- Potter GG, Steffens DC (May 2007). "Contribution of depression to cognitive impairment and dementia in older adults". The Neurologist. 13 (3): 105–17. doi:10.1097/01.nrl.0000252947.15389.a9. PMID 17495754. S2CID 24569198.
- Zhang S, Smailagic N, Hyde C, Noel-Storr AH, Takwoingi Y, McShane R, Feng J (July 2014). "(11)C-PIB-PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI)". The Cochrane Database of Systematic Reviews (7): CD010386. doi:10.1002/14651858.CD010386.pub2. PMC 6464750. PMID 25052054.
- Smailagic N, Vacante M, Hyde C, Martin S, Ukoumunne O, Sachpekidis C (January 2015). "¹⁸F-FDG PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI)". The Cochrane Database of Systematic Reviews. 1: CD010632. doi:10.1002/14651858.CD010632.pub2. PMC 7081123. PMID 25629415.
- Patterson C, Feightner JW, Garcia A, Hsiung GY, MacKnight C, Sadovnick AD (February 2008). "Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease". CMAJ. 178 (5): 548–56. doi:10.1503/cmaj.070796. PMC 2244657. PMID 18299540.
- Rosendorff C, Beeri MS, Silverman JM (2007). "Cardiovascular risk factors for Alzheimer's disease". The American Journal of Geriatric Cardiology. 16 (3): 143–49. doi:10.1111/j.1076-7460.2007.06696.x. PMID 17483665.
- Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, et al. (January 2020). "Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies". The Lancet. Neurology. 19 (1): 61–70. doi:10.1016/S1474-4422(19)30393-X. PMID 31706889.
- Reiss AB, Wirkowski E (2007). "Role of HMG-CoA reductase inhibitors in neurological disorders : progress to date". Drugs. 67 (15): 2111–20. doi:10.2165/00003495-200767150-00001. PMID 17927279.
- Kuller LH (August 2007). "Statins and dementia". Current Atherosclerosis Reports. 9 (2): 154–61. doi:10.1007/s11883-007-0012-9. PMID 17877925.
- McGuinness B, Craig D, Bullock R, Malouf R, Passmore P (July 2014). "Statins for the treatment of dementia". The Cochrane Database of Systematic Reviews. 7 (7): CD007514. doi:10.1002/14651858.CD007514.pub3. PMID 25004278.
- Szekely CA, Town T, Zandi PP (2007). NSAIDs for the chemoprevention of Alzheimer's disease. Subcellular Biochemistry. 42. pp. 229–48. doi:10.1007/1-4020-5688-5_11. ISBN 978-1-4020-5687-1. PMID 17612054.
- Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P (February 2011). "Soothing the inflamed brain: effect of non-steroidal anti-inflammatory drugs on Alzheimer's disease pathology". CNS & Neurological Disorders Drug Targets. 10 (1): 57–67. doi:10.2174/187152711794488665. PMID 21143138.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J (January 2017). "Long-term hormone therapy for perimenopausal and postmenopausal women". The Cochrane Database of Systematic Reviews. 1: CD004143. doi:10.1002/14651858.CD004143.pub5. PMC 6465148. PMID 28093732.
- Stern Y (July 2006). "Cognitive reserve and Alzheimer disease". Alzheimer Disease and Associated Disorders. 20 (3 Suppl 2): S69–74. doi:10.1097/01.wad.0000213815.20177.19. PMID 16917199. S2CID 9827674.
- Paradise M, Cooper C, Livingston G (February 2009). "Systematic review of the effect of education on survival in Alzheimer's disease". International Psychogeriatrics. 21 (1): 25–32. doi:10.1017/S1041610208008053. PMID 19026089.
- Neergaard L (19 February 2011). "Speaking 2 Languages May Delay Getting Alzheimer's". The Denver Post. Associated Press. Archived from the original on 2 May 2014.
- Cheng ST (September 2016). "Cognitive Reserve and the Prevention of Dementia: the Role of Physical and Cognitive Activities". Current Psychiatry Reports. 18 (9): 85. doi:10.1007/s11920-016-0721-2. PMC 4969323. PMID 27481112.
- Farina N, Rusted J, Tabet N (January 2014). "The effect of exercise interventions on cognitive outcome in Alzheimer's disease: a systematic review". International Psychogeriatrics. 26 (1): 9–18. doi:10.1017/S1041610213001385. PMID 23962667. S2CID 24936334.
- Hu N, Yu JT, Tan L, Wang YL, Sun L, Tan L (2013). "Nutrition and the risk of Alzheimer's disease". BioMed Research International (Review). 2013: 1–12. doi:10.1155/2013/524820. PMC 3705810. PMID 23865055.
- Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, Pilotto A (May 2011). "Diet and Alzheimer's disease risk factors or prevention: the current evidence". Expert Review of Neurotherapeutics. 11 (5): 677–708. doi:10.1586/ern.11.56. PMID 21539488.
- Kanoski SE, Davidson TL (April 2011). "Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity". Physiology & Behavior (Review). 103 (1): 59–68. doi:10.1016/j.physbeh.2010.12.003. PMC 3056912. PMID 21167850.
- Solfrizzi V, Capurso C, D'Introno A, Colacicco AM, Santamato A, Ranieri M, Fiore P, Capurso A, Panza F (January 2008). "Lifestyle-related factors in predementia and dementia syndromes". Expert Review of Neurotherapeutics. 8 (1): 133–58. doi:10.1586/14737175.8.1.133. PMID 18088206.
- Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N (2010). "Caffeine intake and dementia: systematic review and meta-analysis". Journal of Alzheimer's Disease. 20 Suppl 1: S187–204. doi:10.3233/JAD-2010-091387. PMID 20182026.
- Nehlig A (March 2013). "The neuroprotective effects of cocoa flavanol and its influence on cognitive performance". British Journal of Clinical Pharmacology (Review). 75 (3): 716–27. doi:10.1111/j.1365-2125.2012.04378.x. PMC 3575938. PMID 22775434.
- Stoclet JC, Schini-Kerth V (March 2011). "[Dietary flavonoids and human health]". Annales Pharmaceutiques Francaises. 69 (2): 78–90. doi:10.1016/j.pharma.2010.11.004. PMID 21440100.
- Ono K, Yamada M (April 2012). "Vitamin A and Alzheimer's disease". Geriatrics & Gerontology International (Review). 12 (2): 180–88. doi:10.1111/j.1447-0594.2011.00786.x. PMID 22221326.
- Lerner AJ, Gustaw-Rothenberg K, Smyth S, Casadesus G (March–April 2012). "Retinoids for treatment of Alzheimer's disease". BioFactors. 38 (2): 84–89. doi:10.1002/biof.196. PMID 22419567.
- Heo JH, Lee KM (March 2013). "The possible role of antioxidant vitamin C in Alzheimer's disease treatment and prevention". American Journal of Alzheimer's Disease and Other Dementias (Review). 28 (2): 120–25. doi:10.1177/1533317512473193. PMID 23307795.
- Boothby LA, Doering PL (December 2005). "Vitamin C and vitamin E for Alzheimer's disease". The Annals of Pharmacotherapy. 39 (12): 2073–80. doi:10.1345/aph.1E495. PMID 16227450.
- Farina N, Llewellyn D, Isaac MG, Tabet N (April 2017). "Vitamin E for Alzheimer's dementia and mild cognitive impairment". The Cochrane Database of Systematic Reviews. 4: CD002854. doi:10.1002/14651858.CD002854.pub5. PMC 6478142. PMID 28418065.
- Loef M, Schrauzer GN, Walach H (2011). "Selenium and Alzheimer's disease: a systematic review". Journal of Alzheimer's Disease (Review). 26 (1): 81–104. doi:10.3233/JAD-2011-110414. PMID 21593562. S2CID 30661765.
- Loef M, von Stillfried N, Walach H (September 2012). "Zinc diet and Alzheimer's disease: a systematic review". Nutritional Neuroscience (Review). 15 (5): 2–12. doi:10.1179/1476830512Y.0000000010. PMID 22583839.
- Avan A, Hoogenraad TU (2015). "Zinc and Copper in Alzheimer's Disease". Journal of Alzheimer's Disease (Review). 46 (1): 89–92. doi:10.3233/JAD-150186. PMID 25835420.
- Malouf R, Grimley Evans J (October 2008). "Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people". The Cochrane Database of Systematic Reviews (4): CD004514. doi:10.1002/14651858.CD004514.pub2. PMID 18843658.
- Wald DS, Kasturiratne A, Simmonds M (June 2010). "Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials". The American Journal of Medicine. 123 (6): 522–527.e2. doi:10.1016/j.amjmed.2010.01.017. PMID 20569758.
- Cunnane SC, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P (January 2013). "Docosahexaenoic acid homeostasis, brain aging and Alzheimer's disease: Can we reconcile the evidence?". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 88 (1): 61–70. doi:10.1016/j.plefa.2012.04.006. PMID 22575581.
- Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A (April 2016). "Omega-3 fatty acids for the treatment of dementia". The Cochrane Database of Systematic Reviews. 4: CD009002. doi:10.1002/14651858.CD009002.pub3. PMC 7117565. PMID 27063583.
- Hamaguchi T, Ono K, Yamada M (October 2010). "REVIEW: Curcumin and Alzheimer's disease". CNS Neuroscience & Therapeutics (review). 16 (5): 285–97. doi:10.1111/j.1755-5949.2010.00147.x. PMC 6493893. PMID 20406252.
- Birks J, Grimley Evans J (January 2009). "Ginkgo biloba for cognitive impairment and dementia". The Cochrane Database of Systematic Reviews (1): CD003120. doi:10.1002/14651858.CD003120.pub3. PMID 19160216.
- Krishnan S, Cairns R, Howard R (April 2009). Krishnan S (ed.). "Cannabinoids for the treatment of dementia". The Cochrane Database of Systematic Reviews (2): CD007204. doi:10.1002/14651858.CD007204.pub2. PMC 7197039. PMID 19370677.
- Bilkei-Gorzo A (December 2012). "The endocannabinoid system in normal and pathological brain ageing". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 367 (1607): 3326–41. doi:10.1098/rstb.2011.0388. PMC 3481530. PMID 23108550.
- Birks JS, Harvey RJ (June 2018). "Donepezil for dementia due to Alzheimer's disease". The Cochrane Database of Systematic Reviews. 6: CD001190. doi:10.1002/14651858.CD001190.pub3. PMC 6513124. PMID 29923184.
- Birks JS, Grimley Evans J (April 2015). Birks JS (ed.). "Rivastigmine for Alzheimer's disease". The Cochrane Database of Systematic Reviews (4): CD001191. doi:10.1002/14651858.CD001191.pub3. PMID 25858345.
- Fink HA, Linskens EJ, MacDonald R, Silverman PC, McCarten JR, Talley KM, et al. (May 2020). "Benefits and Harms of Prescription Drugs and Supplements for Treatment of Clinical Alzheimer-Type Dementia". Annals of Internal Medicine. 172 (10): 656–668. doi:10.7326/M19-3887. PMID 32340037.
- Geula C, Mesulam MM (1995). "Cholinesterases and the pathology of Alzheimer disease". Alzheimer Disease and Associated Disorders. 9 Suppl 2: 23–28. doi:10.1097/00002093-199501002-00005. PMID 8534419.
- Stahl SM (November 2000). "The new cholinesterase inhibitors for Alzheimer's disease, Part 2: illustrating their mechanisms of action". The Journal of Clinical Psychiatry. 61 (11): 813–14. doi:10.4088/JCP.v61n1101. PMID 11105732.
- Birks J (January 2006). Birks J (ed.). "Cholinesterase inhibitors for Alzheimer's disease". The Cochrane Database of Systematic Reviews (1): CD005593. doi:10.1002/14651858.CD005593. PMID 16437532.
- Raschetti R, Albanese E, Vanacore N, Maggini M (November 2007). "Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials". PLOS Medicine. 4 (11): e338. doi:10.1371/journal.pmed.0040338. PMC 2082649. PMID 18044984.
- Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, Williams BR (2013). Applied therapeutics : the clinical use of drugs (10th ed.). Baltimore: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2385. ISBN 978-1-60913-713-7.
- Lipton SA (February 2006). "Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond". Nature Reviews. Drug Discovery. 5 (2): 160–70. doi:10.1038/nrd1958. PMID 16424917.
- "Memantine". US National Library of Medicine (Medline). 4 January 2004. Archived from the original on 22 February 2010. Retrieved 3 February 2010.
- McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, Maayan N, Ware J, Debarros J (May 2019). "Memantine for dementia". The Cochrane Database of Systematic Reviews. 3 (3): CD003154. doi:10.1002/14651858.CD003154.pub6. PMC 6425228. PMID 30891742.
- "Namenda prescribing information" (PDF). Forest Pharmaceuticals. Archived from the original (PDF) on 27 February 2008. Retrieved 19 February 2008. (primary source)
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M (March 2008). "Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline". Annals of Internal Medicine. 148 (5): 379–97. doi:10.7326/0003-4819-148-5-200803040-00009. PMID 18316756.
- Ballard C, Waite J (January 2006). Ballard CG (ed.). "The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease". The Cochrane Database of Systematic Reviews (1): CD003476. doi:10.1002/14651858.CD003476.pub2. PMID 16437455.
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R (February 2009). "The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial". The Lancet Neurology. 8 (2): 151–57. doi:10.1016/S1474-4422(08)70295-3. PMID 19138567. Lay summary.
- Declercq T, Petrovic M, Azermai M, Vander Stichele R, De Sutter AI, van Driel ML, Christiaens T (March 2013). "Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia" (PDF). The Cochrane Database of Systematic Reviews. 3 (3): CD007726. doi:10.1002/14651858.CD007726.pub2. hdl:1854/LU-3109108. PMID 23543555.
- Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, Tariot PN, et al. (Steering Committee on Practice Guidelines) (December 2007). "American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition". The American Journal of Psychiatry. 164 (12 Suppl): 5–56. PMID 18340692.
- Bottino CM, Carvalho IA, Alvarez AM, Avila R, Zukauskas PR, Bustamante SE, et al. (December 2005). "Cognitive rehabilitation combined with drug treatment in Alzheimer's disease patients: a pilot study". Clinical Rehabilitation. 19 (8): 861–69. doi:10.1191/0269215505cr911oa. PMID 16323385.
- Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. (May 2001). "Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 56 (9): 1154–66. doi:10.1212/WNL.56.9.1154. PMID 11342679.
- Hermans DG, Htay UH, McShane R (January 2007). "Non-pharmacological interventions for wandering of people with dementia in the domestic setting". The Cochrane Database of Systematic Reviews (1): CD005994. doi:10.1002/14651858.CD005994.pub2. PMC 6669244. PMID 17253573.
- Robinson L, Hutchings D, Dickinson HO, Corner L, Beyer F, Finch T, Hughes J, Vanoli A, Ballard C, Bond J (January 2007). "Effectiveness and acceptability of non-pharmacological interventions to reduce wandering in dementia: a systematic review". International Journal of Geriatric Psychiatry. 22 (1): 9–22. doi:10.1002/gps.1643. PMID 17096455.
- Abraha I, Rimland JM, Trotta FM, Dell'Aquila G, Cruz-Jentoft A, Petrovic M, Gudmundsson A, Soiza R, O'Mahony D, Guaita A, Cherubini A (March 2017). "Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series". BMJ Open. 7 (3): e012759. doi:10.1136/bmjopen-2016-012759. PMC 5372076. PMID 28302633.
- Chung JC, Lai CK, Chung PM, French HP (2002). "Snoezelen for dementia". The Cochrane Database of Systematic Reviews (4): CD003152. doi:10.1002/14651858.CD003152. PMID 12519587.
- Woods B, O'Philbin L, Farrell EM, Spector AE, Orrell M (March 2018). "Reminiscence therapy for dementia". The Cochrane Database of Systematic Reviews. 3: CD001120. doi:10.1002/14651858.CD001120.pub3. PMC 6494367. PMID 29493789.
- Zetteler J (November 2008). "Effectiveness of simulated presence therapy for individuals with dementia: a systematic review and meta-analysis". Aging & Mental Health. 12 (6): 779–85. doi:10.1080/13607860802380631. PMID 19023729.
- Neal M, Barton Wright P (2003). Neal M (ed.). "Validation therapy for dementia". The Cochrane Database of Systematic Reviews (3): CD001394. doi:10.1002/14651858.CD001394. PMID 12917907.
- Chung JC, Lai CK, Chung PM, French HP (2002). Chung JC (ed.). "Snoezelen for dementia". The Cochrane Database of Systematic Reviews (4): CD003152. doi:10.1002/14651858.CD003152. PMID 12519587. (up to date as of 2009)
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, Orrell M (September 2003). "Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial". The British Journal of Psychiatry. 183 (3): 248–54. doi:10.1192/bjp.183.3.248. PMID 12948999.
- Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck WW (February 2001). "A randomized, controlled trial of a home environmental intervention: effect on efficacy and upset in caregivers and on daily function of persons with dementia". The Gerontologist. 41 (1): 4–14. doi:10.1093/geront/41.1.4. PMID 11220813.
- Gitlin LN, Hauck WW, Dennis MP, Winter L (March 2005). "Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer's disease and related disorders". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 60 (3): 368–74. doi:10.1093/gerona/60.3.368. PMID 15860476.
- "Treating Behavioral and Psychiatric Symptoms". Alzheimer's Association. 2006. Archived from the original on 25 September 2006. Retrieved 25 September 2006.
- Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A (August 2004). "Visual contrast enhances food and liquid intake in advanced Alzheimer's disease". Clinical Nutrition. 23 (4): 533–38. doi:10.1016/j.clnu.2003.09.015. PMID 15297089.
- Dudek SB (2007). Nutrition Essentials for Nursing Practice. Hagerstown, Maryland: Lippincott Williams & Wilkins. p. 360. ISBN 978-0-7817-6651-7. Retrieved 19 August 2008.
- Dennehy C (2006). "Analysis of patients' rights: dementia and PEG insertion". British Journal of Nursing. 15 (1): 18–20. doi:10.12968/bjon.2006.15.1.20303. PMID 16415742.
- Chernoff R (April 2006). "Tube feeding patients with dementia". Nutrition in Clinical Practice. 21 (2): 142–46. doi:10.1177/0115426506021002142. PMID 16556924.
- Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R (July 1999). "Predictors of mortality in patients with Alzheimer's disease living in nursing homes". Journal of Neurology, Neurosurgery, and Psychiatry. 67 (1): 59–65. doi:10.1136/jnnp.67.1.59. PMC 1736445. PMID 10369823.
- Medical issues:
- Head B (January 2003). "Palliative care for persons with dementia". Home Healthcare Nurse. 21 (1): 53–60, quiz 61. doi:10.1097/00004045-200301000-00012. PMID 12544465.
- Friedlander AH, Norman DC, Mahler ME, Norman KM, Yagiela JA (September 2006). "Alzheimer's disease: psychopathology, medical management and dental implications". Journal of the American Dental Association. 137 (9): 1240–51. doi:10.14219/jada.archive.2006.0381. PMID 16946428.
- Belmin J (2007). "Practical guidelines for the diagnosis and management of weight loss in Alzheimer's disease: a consensus from appropriateness ratings of a large expert panel". The Journal of Nutrition, Health & Aging. 11 (1): 33–37. PMID 17315078.
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello M, Teri L (October 2003). "Training caregivers to change the sleep hygiene practices of patients with dementia: the NITE-AD project". Journal of the American Geriatrics Society. 51 (10): 1455–60. doi:10.1046/j.1532-5415.2003.51466.x. PMID 14511168.
- Perls TT, Herget M (December 1995). "Higher respiratory infection rates on an Alzheimer's special care unit and successful intervention". Journal of the American Geriatrics Society. 43 (12): 1341–44. doi:10.1111/j.1532-5415.1995.tb06611.x. PMID 7490383.
- Shega JW, Levin A, Hougham GW, Cox-Hayley D, Luchins D, Hanrahan P, Stocking C, Sachs GA (April 2003). "Palliative Excellence in Alzheimer Care Efforts (PEACE): a program description". Journal of Palliative Medicine. 6 (2): 315–20. doi:10.1089/109662103764978641. PMID 12854952. S2CID 6072807.
- Zanetti O, Solerte SB, Cantoni F (2009). "Life expectancy in Alzheimer's disease (AD)". Archives of Gerontology and Geriatrics. 49 Suppl 1: 237–43. doi:10.1016/j.archger.2009.09.035. PMID 19836639.
- Mölsä PK, Marttila RJ, Rinne UK (March 1995). "Long-term survival and predictors of mortality in Alzheimer's disease and multi-infarct dementia". Acta Neurologica Scandinavica. 91 (3): 159–64. doi:10.1111/j.1600-0404.1995.tb00426.x. PMID 7793228.
- Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, Larson EB (August 1996). "Predictors of mortality in patients diagnosed with probable Alzheimer's disease". Neurology. 47 (2): 433–39. doi:10.1212/wnl.47.2.433. PMID 8757016.
- Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA (April 2004). "Survival after initial diagnosis of Alzheimer disease". Annals of Internal Medicine. 140 (7): 501–09. doi:10.7326/0003-4819-140-7-200404060-00008. PMID 15068977.
- Jagger C, Clarke M, Stone A (January 1995). "Predictors of survival with Alzheimer's disease: a community-based study". Psychological Medicine. 25 (1): 171–77. doi:10.1017/S0033291700028191. PMID 7792352.
- Dodge HH, Shen C, Pandav R, DeKosky ST, Ganguli M (February 2003). "Functional transitions and active life expectancy associated with Alzheimer disease". Archives of Neurology. 60 (2): 253–59. doi:10.1001/archneur.60.2.253. PMID 12580712.
- Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST (May 2005). "Alzheimer disease and mortality: a 15-year epidemiological study". Archives of Neurology. 62 (5): 779–84. doi:10.1001/archneur.62.5.779. PMID 15883266.
- Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Román GC (January 2008). "Incidence and subtypes of dementia in three elderly populations of central Spain". Journal of the Neurological Sciences. 264 (1–2): 63–72. doi:10.1016/j.jns.2007.07.021. PMID 17727890.
- Di Carlo A, Baldereschi M, Amaducci L, Lepore V, Bracco L, Maggi S, Bonaiuto S, Perissinotto E, Scarlato G, Farchi G, Inzitari D (January 2002). "Incidence of dementia, Alzheimer's disease, and vascular dementia in Italy. The ILSA Study". Journal of the American Geriatrics Society. 50 (1): 41–48. doi:10.1046/j.1532-5415.2002.50006.x. PMID 12028245.
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, et al. (December 1999). "Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group". Neurology. 53 (9): 1992–97. doi:10.1212/wnl.53.9.1992. PMID 10599770.
- Tejada-Vera B. (2013). Mortality from Alzheimer's Disease in the United States: Data for 2000 and 2010. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics.
- 2000 U.S. estimates:
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA (August 2003). "Alzheimer disease in the US population: prevalence estimates using the 2000 census". Archives of Neurology. 60 (8): 1119–22. doi:10.1001/archneur.60.8.1119. PMID 12925369.
- "Profiles of General Demographic Characteristics, 2000 Census of Population and Housing, United States" (PDF). U.S. Census Bureau. 2001. Archived from the original (PDF) on 19 August 2008. Retrieved 27 August 2008.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (December 2005). "Global prevalence of dementia: a Delphi consensus study". Lancet. 366 (9503): 2112–17. doi:10.1016/S0140-6736(05)67889-0. PMC 2850264. PMID 16360788.
- World Health Organization (2006). Neurological Disorders: Public Health Challenges. Switzerland: World Health Organization. pp. 204–07. ISBN 978-92-4-156336-9. Archived from the original on 10 February 2010.
-
2006 prevalence estimate:
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (July 2007). "Forecasting the global burden of Alzheimer's disease". Alzheimer's & Dementia. 3 (3): 186–91. CiteSeerX 10.1.1.729.847. doi:10.1016/j.jalz.2007.04.381. PMID 19595937. Archived from the original on 7 December 2008. Retrieved 18 June 2008.
- "Highlights" (PDF). World Population Prospects: The 2006 Revision. Working Paper No. ESA/P/WP.202. Population Division, Department of Economic and Social Affairs, United Nations. 2007. Archived from the original (PDF) on 19 August 2008. Retrieved 27 August 2008.
- Auguste D.:
- Alzheimer A (1907). "Über eine eigenartige Erkrankung der Hirnrinde" [About a peculiar disease of the cerebral cortex]. Allgemeine Zeitschrift für Psychiatrie und Psychisch-Gerichtlich Medizin (in German). 64 (1–2): 146–48.
- Alzheimer A (1987). Translated by H. Greenson. "About a peculiar disease of the cerebral cortex. By Alois Alzheimer, 1907 (Translated by L. Jarvik and H. Greenson)". Alzheimer Disease and Associated Disorders. 1 (1): 3–8. PMID 3331112.
- Ulrike M, Konrad M (2003). Alzheimer: The Life of a Physician and the Career of a Disease. New York: Columbia University Press. p. 270. ISBN 978-0-231-11896-5.
- Berrios GE (1990). "Alzheimer's Disease: A Conceptual History". Int. J. Geriatr. Psychiatry. 5 (6): 355–65. doi:10.1002/gps.930050603.
- Kraepelin Emil (2007). Clinical Psychiatry: A Textbook For Students And Physicians (Reprint). Translated by Diefendorf A. Ross. Kessinger Publishing. p. 568. ISBN 978-1-4325-0833-3.
- Katzman R, Terry RD, Bick KL, eds. (1978). Alzheimer's Disease: Senile Dementia and Related Disorders. New York: Raven Press. p. 595. ISBN 978-0-89004-225-0.
- Boller F, Forbes MM (June 1998). "History of dementia and dementia in history: an overview". Journal of the Neurological Sciences. 158 (2): 125–33. doi:10.1016/S0022-510X(98)00128-2. PMID 9702682.
- Amaducci LA, Rocca WA, Schoenberg BS (November 1986). "Origin of the distinction between Alzheimer's disease and senile dementia: how history can clarify nosology". Neurology. 36 (11): 1497–9. doi:10.1212/wnl.36.11.1497. PMID 3531918.
- Allegri RF, Butman J, Arizaga RL, Machnicki G, Serrano C, Taragano FE, Sarasola D, Lon L (August 2007). "Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina". International Psychogeriatrics. 19 (4): 705–18. doi:10.1017/S1041610206003784. PMID 16870037. S2CID 41247271.
- Suh GH, Knapp M, Kang CJ (August 2006). "The economic costs of dementia in Korea, 2002". International Journal of Geriatric Psychiatry. 21 (8): 722–28. doi:10.1002/gps.1552. PMID 16858741.
- Wimo A, Jonsson L, Winblad B (2006). "An estimate of the worldwide prevalence and direct costs of dementia in 2003". Dementia and Geriatric Cognitive Disorders. 21 (3): 175–81. doi:10.1159/000090733. PMID 16401889.
- Moore MJ, Zhu CW, Clipp EC (July 2001). "Informal costs of dementia care: estimates from the National Longitudinal Caregiver Study". The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 56 (4): S219–28. doi:10.1093/geronb/56.4.S219. PMID 11445614.
- Jönsson L, Eriksdotter Jönhagen M, Kilander L, Soininen H, Hallikainen M, Waldemar G, et al. (May 2006). "Determinants of costs of care for patients with Alzheimer's disease". International Journal of Geriatric Psychiatry. 21 (5): 449–59. doi:10.1002/gps.1489. PMID 16676288.
- "The MetLife study of Alzheimer's disease: The caregiving experience" (PDF). MetLife Mature Market Institute. August 2006. Archived from the original (PDF) on 8 January 2011. Retrieved 5 February 2011.
- Schneider J, Murray J, Banerjee S, Mann A (August 1999). "EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer's disease: I – Factors associated with carer burden". International Journal of Geriatric Psychiatry. 14 (8): 651–61. doi:10.1002/(SICI)1099-1166(199908)14:8<651::AID-GPS992>3.0.CO;2-B. PMID 10489656.
- Murray J, Schneider J, Banerjee S, Mann A (August 1999). "EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer's disease: II – A qualitative analysis of the experience of caregiving". International Journal of Geriatric Psychiatry. 14 (8): 662–67. doi:10.1002/(SICI)1099-1166(199908)14:8<662::AID-GPS993>3.0.CO;2-4. PMID 10489657.
- Zhu CW, Sano M (2006). "Economic considerations in the management of Alzheimer's disease". Clinical Interventions in Aging. 1 (2): 143–54. doi:10.2147/ciia.2006.1.2.143. PMC 2695165. PMID 18044111.
- Gaugler JE, Kane RL, Kane RA, Newcomer R (April 2005). "Early community-based service utilization and its effects on institutionalization in dementia caregiving". The Gerontologist. 45 (2): 177–85. doi:10.1093/geront/45.2.177. PMID 15799982.
- Ritchie K, Lovestone S (November 2002). "The dementias". Lancet. 360 (9347): 1759–66. doi:10.1016/S0140-6736(02)11667-9. PMID 12480441.
- Brodaty H, Hadzi-Pavlovic D (September 1990). "Psychosocial effects on carers of living with persons with dementia". The Australian and New Zealand Journal of Psychiatry. 24 (3): 351–61. doi:10.3109/00048679009077702. PMID 2241719.
- Donaldson C, Tarrier N, Burns A (April 1998). "Determinants of carer stress in Alzheimer's disease". International Journal of Geriatric Psychiatry. 13 (4): 248–56. doi:10.1002/(SICI)1099-1166(199804)13:4<248::AID-GPS770>3.0.CO;2-0. PMID 9646153.
- Pusey H, Richards D (May 2001). "A systematic review of the effectiveness of psychosocial interventions for carers of people with dementia". Aging & Mental Health. 5 (2): 107–19. doi:10.1080/13607860120038302. PMID 11511058.
- Bayley J (2000). Iris: A Memoir of Iris Murdoch. London: Abacus. ISBN 978-0-349-11215-2. OCLC 41960006.
- Sparks N (1996). The notebook. Thorndike, Maine: Thorndike Press. p. 268. ISBN 978-0-7862-0821-0.
- "Thanmathra". Webindia123.com. Archived from the original on 6 November 2007. Retrieved 24 January 2008.
- Ogiwara H (2004). Ashita no Kioku (in Japanese). Tōkyō: Kōbunsha. ISBN 978-4-334-92446-1. OCLC 57352130.
- Munro A (2001). Hateship, Friendship, Courtship, Loveship, Marriage: Stories. New York: A.A. Knopf. ISBN 978-0-375-41300-1. OCLC 46929223.
- "Malcolm and Barbara: A love story". Dfgdocs. Archived from the original on 24 May 2008. Retrieved 24 January 2008.
- "Malcolm and Barbara: A love story". BBC Cambridgeshire. Archived from the original on 10 November 2012. Retrieved 2 March 2008.
- Plunkett J (7 August 2007). "Alzheimer's film-maker to face ITV lawyers". London: Guardian Media. Archived from the original on 15 January 2008. Retrieved 24 January 2008.
- Cummings JL, Morstorf T, Zhong K (July 2014). "Alzheimer's disease drug-development pipeline: few candidates, frequent failures". Alzheimer's Research & Therapy. 6 (4): 37. doi:10.1186/alzrt269. PMC 4095696. PMID 25024750.
- Gutis, Phillip S. (22 March 2019). "An Alzheimer's Drug Trial Gave Me Hope, and Then It Ended". The New York Times. Retrieved 25 March 2019.
- Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ (November 2002). "New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease". The Journal of Biological Chemistry. 277 (45): 42881–90. doi:10.1074/jbc.M206593200. PMID 12167652.
- Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, Oertel W (March 2010). "Intravenous immunoglobulins as a treatment for Alzheimer's disease: rationale and current evidence". Drugs. 70 (5): 513–28. doi:10.2165/11533070-000000000-00000. PMID 20329802. Archived from the original on 17 September 2011.
- Vaccination:
- Hawkes CA, McLaurin J (November 2007). "Immunotherapy as treatment for Alzheimer's disease". Expert Review of Neurotherapeutics. 7 (11): 1535–48. doi:10.1586/14737175.7.11.1535. PMID 17997702.
- Solomon B (June 2007). "Clinical immunologic approaches for the treatment of Alzheimer's disease". Expert Opinion on Investigational Drugs. 16 (6): 819–28. doi:10.1517/13543784.16.6.819. PMID 17501694.
- Woodhouse A, Dickson TC, Vickers JC (2007). "Vaccination strategies for Alzheimer's disease: A new hope?". Drugs & Aging. 24 (2): 107–19. doi:10.2165/00002512-200724020-00003. PMID 17313199.
- "Study Evaluating ACC-001 in Mild to Moderate Alzheimers Disease Subjects". Clinical Trial. US National Institutes of Health. 11 March 2008. Archived from the original on 30 July 2013. Retrieved 5 June 2008.
- "Study Evaluating Safety, Tolerability, and Immunogenicity of ACC-001 in Subjects with Alzheimer's Disease". US National Institutes of Health. Archived from the original on 29 October 2008. Retrieved 5 June 2008.
- "Alzheimer's Disease Vaccine Trial Suspended on Safety Concern". Medpage Today. 18 April 2008. Archived from the original on 23 April 2008. Retrieved 14 June 2008.
- "Bapineuzumab in Patients with Mild to Moderate Alzheimer's Disease/ Apo_e4 Non-carriers" (Clinical Trial). US National Institutes of Health. 29 February 2008. Archived from the original on 22 March 2008. Retrieved 23 March 2008.
- Sperling RA, Jack CR, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ (July 2011). "Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup". Alzheimer's & Dementia. 7 (4): 367–85. doi:10.1016/j.jalz.2011.05.2351. PMC 3693547. PMID 21784348.
- "Safety, Tolerability and Efficacy Study to Evaluate Subjects with Mild Cognitive Impairment" (Clinical Trial). US National Institutes of Health. 11 March 2008. Archived from the original on 22 October 2008. Retrieved 23 March 2008.
- "Study Evaluating the Safety, Tolerability and Efficacy of PBT2 in Patients with Early Alzheimer's Disease" (Clinical Trial). US National Institutes of Health. 13 January 2008. Archived from the original on 31 August 2008. Retrieved 23 March 2008.
- Etanercept research:
- Tobinick E (September 2009). "Tumour necrosis factor modulation for treatment of Alzheimer's disease: rationale and current evidence". CNS Drugs. 23 (9): 713–25. doi:10.2165/11310810-000000000-00000. PMID 19689163.
- Tobinick E, Gross H, Weinberger A, Cohen H (April 2006). "TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study". MedGenMed. 8 (2): 25. PMC 1785182. PMID 16926764.
- Griffin WS (January 2008). "Perispinal etanercept: potential as an Alzheimer therapeutic". Journal of Neuroinflammation. 5: 3. doi:10.1186/1742-2094-5-3. PMC 2241592. PMID 18186919.
- Tobinick E (December 2007). "Perispinal etanercept for treatment of Alzheimer's disease". Current Alzheimer Research. 4 (5): 550–52. doi:10.2174/156720507783018217. PMID 18220520.
- Cheng X, Shen Y, Li R (November 2014). "Targeting TNF: a therapeutic strategy for Alzheimer's disease". Drug Discovery Today. 19 (11): 1822–27. doi:10.1016/j.drudis.2014.06.029. PMID 24998784.
- Wischik CM, Bentham P, Wischik DJ, Seng KM (July 2008). "Tau aggregation inhibitor (TAI) therapy with remberTM arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks". Alzheimer's & Dementia. 4 (4): T167. doi:10.1016/j.jalz.2008.05.438. Retrieved 30 July 2008.
- Harrington C, Rickard J, Horsley D (July 2008). "Methylthioninium chloride (MTC) acts as a tau aggregation inhibitor (TAI) in a cellular model and reverses tau pathology in transgenic mouse models of Alzheimer's disease". Alzheimer's & Dementia. 4 (4): T120–21. doi:10.1016/j.jalz.2008.05.259.
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D (July 2008). "Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study". Lancet. 372 (9634): 207–15. doi:10.1016/S0140-6736(08)61074-0. PMID 18640457.
- Bezprozvanny I (October 2010). "The rise and fall of Dimebon". Drug News & Perspectives (Original article). 23 (8): 518–23. doi:10.1358/dnp.2010.23.8.1500435. PMC 3922928. PMID 21031168.
- "Pfizer And Medivation announce results from two phase 3 studies in Dimebon (latrepirdine*) Alzheimer's disease clinical development program (NASDAQ:MDVN)" (Press release). Archived from the original on 4 September 2012. Retrieved 16 November 2012.
- Wendler A, Wehling M (March 2012). "Translatability scoring in drug development: eight case studies". Journal of Translational Medicine. 10 (1): 39. doi:10.1186/1479-5876-10-39. PMC 3330010. PMID 22397594.
- Baddeley TC, McCaffrey J, Storey JM, Cheung JK, Melis V, Horsley D, Harrington CR, Wischik CM (January 2015). "Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer's disease". The Journal of Pharmacology and Experimental Therapeutics. 352 (1): 110–18. doi:10.1124/jpet.114.219352. PMID 25320049.
- Wischik CM, Harrington CR, Storey JM (April 2014). "Tau-aggregation inhibitor therapy for Alzheimer's disease". Biochemical Pharmacology. 88 (4): 529–39. doi:10.1016/j.bcp.2013.12.008. PMID 24361915.
- Feuerstein A (14 February 2017). "Merck Alzheimer's Drug Study Halted Early for Futility". New York City: TheStreet, Inc. Archived from the original on 16 February 2017.Merck Alzheimer's Drug Study Halted Early for Futility Independent study monitors concluded that there was "virtually no chance of finding a positive clinical effect."
- "After A Big Failure, Scientists And Patients Hunt For A New Type Of Alzheimer's Drug". NPR.org. Retrieved 17 May 2019.
- Gallagher, James (2 May 2019). "Dementia is 'greatest health challenge'". Retrieved 17 May 2019.
- "Biogen Plans Regulatory Filing for Aducanumab in Alzheimer's Disease Based on New Analysis of Larger Dataset from Phase 3 Studies". 22 October 2019.
- Morley JE, Armbrecht HJ, Farr SA, Kumar VB (May 2012). "The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer's disease". Biochimica et Biophysica Acta. 1822 (5): 650–6. doi:10.1016/j.bbadis.2011.11.015. PMID 22142563.
- Marciniak R, Sheardova K, Cermáková P, Hudeček D, Sumec R, Hort J (2014). "Effect of meditation on cognitive functions in context of aging and neurodegenerative diseases". Frontiers in Behavioral Neuroscience. 8: 17. doi:10.3389/fnbeh.2014.00017. PMC 3903052. PMID 24478663.
- Larouche E, Hudon C, Goulet S (January 2015). "Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer's disease: an interdisciplinary perspective". Behavioural Brain Research. 276 (276): 199–212. doi:10.1016/j.bbr.2014.05.058. PMID 24893317.
- Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, et al. (September 2015). "Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy". Nature. 525 (7568): 247–50. Bibcode:2015Natur.525..247J. doi:10.1038/nature15369. PMID 26354483.
- Abbott A (September 2015). "Autopsies reveal signs of Alzheimer's in growth-hormone patients". Nature. 525 (7568): 165–66. Bibcode:2015Natur.525..165A. doi:10.1038/525165a. PMID 26354460.
- Martin C, Solís L, Concha MI, Otth C (June 2011). "[Herpes simplex virus type 1 as risk factor associated to Alzheimer disease]" [Herpes Simplex Virus Type 1 as Risk Factor Associated to Alzheimer Disease]. Revista Médica de Chile (in Spanish). 139 (6): 779–86. doi:10.4067/S0034-98872011000600013. PMID 22051760.
- Wozniak MA, Mee AP, Itzhaki RF (January 2009). "Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques". The Journal of Pathology (Original study). 217 (1): 131–38. doi:10.1002/path.2449. PMID 18973185.
- Itzhaki RF (2014). "Herpes simplex virus type 1 and Alzheimer's disease: increasing evidence for a major role of the virus". Frontiers in Aging Neuroscience. 6: 202. doi:10.3389/fnagi.2014.00202. PMC 4128394. PMID 25157230.
- Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, et al. (2016). "Microbes and Alzheimer's Disease". Journal of Alzheimer's Disease. 51 (4): 979–84. doi:10.3233/JAD-160152. PMC 5457904. PMID 26967229. Archived from the original on 10 November 2016.
- Alonso R, Pisa D, Rábano A, Carrasco L (July 2014). "Alzheimer's disease and disseminated mycoses". European Journal of Clinical Microbiology & Infectious Diseases. 33 (7): 1125–32. doi:10.1007/s10096-013-2045-z. PMID 24452965.
- Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L (October 2015). "Different Brain Regions are Infected with Fungi in Alzheimer's Disease". Scientific Reports. 5: 15015. Bibcode:2015NatSR...515015P. doi:10.1038/srep15015. PMC 4606562. PMID 26468932.
- "Fungus, the bogeyman". The Economist. 22 October 2015. Archived from the original on 8 August 2017.
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD (May 2016). "Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease". Science Translational Medicine. 8 (340): 340ra72. doi:10.1126/scitranslmed.aaf1059. PMC 5505565. PMID 27225182.
- Kolata, Gina (25 May 2016). "Could Alzheimer's Stem From Infections? It Makes Sense, Experts Say". The New York Times. Archived from the original on 4 February 2017.
- "Alzheimer's culprit may fight other diseases". Science News. 16 June 2016. Archived from the original on 26 May 2016.
- Dougall NJ, Bruggink S, Ebmeier KP (2004). "Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia". The American Journal of Geriatric Psychiatry. 12 (6): 554–70. doi:10.1176/appi.ajgp.12.6.554. PMID 15545324.
- Carpenter AP, Pontecorvo MJ, Hefti FF, Skovronsky DM (August 2009). "The use of the exploratory IND in the evaluation and development of 18F-PET radiopharmaceuticals for amyloid imaging in the brain: a review of one company's experience". The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 53 (4): 387–93. PMID 19834448.
- Leung K (8 April 2010). "(E)-4-(2-(6-(2-(2-(2-(18F-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methyl benzenamine [[18F]AV-45]". Molecular Imaging and Contrast Agent Database. Archived from the original on 7 June 2010. Retrieved 24 June 2010.
- Rabinovici GD, Jagust WJ (2009). "Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo". Behavioural Neurology. 21 (1): 117–28. doi:10.1155/2009/609839. PMC 2804478. PMID 19847050. Archived from the original on 30 July 2013.
- O'Brien JT (December 2007). "Role of imaging techniques in the diagnosis of dementia". The British Journal of Radiology. 80 Spec No 2 (Spec No 2): S71–77. doi:10.1259/bjr/33117326. PMID 18445747.
- "FDA Panel Recommends Conditional Approval for PET Agent". Imaging Technology News. 21 January 2011. Retrieved 17 June 2019.
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. (January 2011). "Use of florbetapir-PET for imaging beta-amyloid pathology". JAMA. 305 (3): 275–83. doi:10.1001/jama.2010.2008. PMC 7041965. PMID 21245183.
- "Amyvid". Community register of medicinal products for human use. European Community. 17 January 2013. Retrieved 18 April 2018.
- Chong MS, Sahadevan S (September 2005). "Preclinical Alzheimer's disease: diagnosis and prediction of progression". The Lancet. Neurology. 4 (9): 576–79. doi:10.1016/s1474-4422(05)70168-x. PMID 16109364.
- Sharma N, Singh AN (July 2016). "Exploring Biomarkers for Alzheimer's Disease". Journal of Clinical and Diagnostic Research (Review). 10 (7): KE01–06. doi:10.7860/JCDR/2016/18828.8166. PMC 5020308. PMID 27630867.
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. (January 2015). "Blood-brain barrier breakdown in the aging human hippocampus". Neuron. 85 (2): 296–302. doi:10.1016/j.neuron.2014.12.032. PMID 25611508.
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, et al. (February 2019). "Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction". Nature Medicine. 25 (2): 270–276. doi:10.1038/s41591-018-0297-y. PMID 30643288.
- Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. (May 2020). "APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline". Nature. 581 (7806): 71–76. doi:10.1038/s41586-020-2247-3. PMID 32376954.
Further reading
| Library resources about Alzheimer's Disease |
- Irvine K, Laws KR, Gale TM, Kondel TK (2012). "Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis". Journal of Clinical and Experimental Neuropsychology (Meta-analysis). 34 (9): 989–98. doi:10.1080/13803395.2012.712676. PMID 22913619.
- Harilal S, Jose J, Parambi DG, Kumar R, Mathew GE, Uddin MS, et al. (September 2019). "Advancements in nanotherapeutics for Alzheimer's disease: current perspectives". The Journal of Pharmacy and Pharmacology. 71 (9): 1370–1383. doi:10.1111/jphp.13132. PMID 31304982.
External links
| Wikimedia Commons has media related to Alzheimer's disease. |
| Classification | |
|---|---|
| External resources |