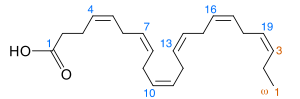
Docosahexaenoic acid
Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. In physiological literature, it is given the name 22:6(n-3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk (breast milk), fish oil, or algae oil.[1]
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.118.398 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C22H32O2 | |||
| Molar mass | 328.488 g/mol | ||
| Density | 0.943 g/cm3 | ||
| Melting point | −44 °C (−47 °F; 229 K) | ||
| Boiling point | 446.7 °C (836.1 °F; 719.8 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
DHA's structure is a carboxylic acid (-oic acid) with a 22-carbon chain (docosa- derives from the Ancient Greek for 22) and six (hexa-) cis double bonds (-en-);[2] with the first double bond located at the third carbon from the omega end.[3] Its trivial name is cervonic acid, its systematic name is all-cis-docosa-4,7,10,13,16,19-hexa-enoic acid, and its shorthand name is 22:6(n−3) in the nomenclature of fatty acids.
Most of the DHA in fish and multi-cellular organisms with access to cold-water oceanic foods originates from photosynthetic and heterotrophic microalgae, and becomes increasingly concentrated in organisms the further they are up the food chain. DHA is also commercially manufactured from microalgae: Crypthecodinium cohnii and another of the genus Schizochytrium.[4] DHA manufactured using microalgae is vegetarian.[5]
In organisms that do not eat algae containing DHA nor animal products containing DHA, DHA is instead produced internally from α-linolenic acid, a shorter omega-3 fatty acid manufactured by plants (and also occurring in animal products as obtained from plants).[6] Limited amounts of eicosapentaenoic and docosapentaenoic acids are possible products of α-linolenic acid metabolism in young women[7] and men.[6] DHA in breast milk is important for the developing infant.[8] Rates of DHA production in women are 15% higher than in men.[9]
DHA is a major fatty acid in brain phospholipids and the retina. While the potential roles of DHA in the mechanisms of Alzheimer's disease are under active research,[10] studies of fish oil supplements, which contain DHA, have failed to support claims of preventing cardiovascular diseases.[11][12][13]
Central nervous system constituent
DHA is the most abundant omega-3 fatty acid in the brain and retina.[14] DHA comprises 40% of the polyunsaturated fatty acids (PUFAs) in the brain and 60% of the PUFAs in the retina. Fifty percent of a neuronal plasma membrane is composed of DHA.[15] DHA modulates the carrier-mediated transport of choline, glycine, and taurine, the function of delayed rectifier potassium channels, and the response of rhodopsin contained in the synaptic vesicles.[16][17]
Phosphatidylserine (PS) – which contains high DHA content – has roles in neuronal signaling and neurotransmitter synthesis,[14] and DHA deficiency is associated with cognitive decline.[14][18] DHA levels are reduced in the brain tissue of severely depressed people.[19][20]
Metabolic synthesis
In humans, DHA is either obtained from the diet or may be converted in small amounts from eicosapentaenoic acid (EPA, 20:5, ω-3) via docosapentaenoic acid (DPA, 22:5 ω-3) as an intermediate.[7][6] This synthesis had been thought to occur through an elongation step followed by the action of Δ4-desaturase.[6] It is now considered more likely that DHA is biosynthesized via a C24 intermediate followed by beta oxidation in peroxisomes. Thus, EPA is twice elongated, yielding 24:5 ω-3, then desaturated to 24:6 ω-3, then shortened to DHA (22:6 ω-3) via beta oxidation. This pathway is known as "Sprecher's shunt".[21][22]
In organisms such as microalgae, mosses and fungi, biosynthesis of DHA usually occurs as a series of desaturation and elongation reactions, catalyzed by the sequential action of desaturase and elongase enzymes. One known pathway in these organisms involves:
- a desaturation at the sixth carbon of alpha-linolenic acid by a Δ6 desaturase to produce stearidonic acid,
- elongation of the stearidonic acid by a Δ6 elongase to produce to eicosatetraenoic acid,
- desaturation at the fifth carbon of eicosatetraenoic acid by a Δ5 desaturase to produce eicosapentaenoic acid,
- elongation of eicosapentaenoic acid by a Δ5 elongase to produce docosapentaenoic acid, and
- desaturation at the fourth carbon of docosapentaenoic acid by a Δ4 desaturase to produce DHA.[23]
Metabolism
DHA can be metabolized into DHA-derived specialized pro-resolving mediators (SPMs), DHA epoxides, electrophilic oxo-derivatives (EFOX) of DHA, neuroprostanes, ethanolamines, acylglycerols, docosahexaenoyl amides of amino acids or neurotransmitters, and branched DHA esters of hydroxy fatty acids, among others.[24]
The enzyme CYP2C9 metabolizes DHA to epoxydocosapentaenoic acids (EDPs; primarily 19,20-epoxy-eicosapentaenoic acid isomers [i.e. 10,11-EDPs]).[25]
Potential health effects
Pregnancy and lactation
Foods high in omega-3 fatty acids may be recommended to women who want to become pregnant or when nursing.[26] A working group from the International Society for the Study of Fatty Acids and Lipids recommended 300 mg/day of DHA for pregnant and lactating women, whereas the average consumption was between 45 mg and 115 mg per day of the women in the study, similar to a Canadian study.[27]
Brain and visual functions
A major structural component of the mammalian central nervous system, DHA is the most abundant omega−3 fatty acid in the brain and retina.[28] Brain and retinal function rely on dietary intake of DHA to support a broad range of cell membrane and cell signaling properties, particularly in grey matter and retinal photoreceptor cell outer segments, which are rich in membranes.[29][30]
A systematic review found that DHA had no significant benefits in improving visual field in individuals with retinitis pigmentosa.[31]
Nutrition

Ordinary types of cooked salmon contain 500–1500 mg DHA and 300–1000 mg EPA per 100 grams.[32] Additional rich seafood sources of DHA include caviar (3400 mg per 100 grams), anchovies (1292 mg per 100 grams), mackerel (1195 mg per 100 grams), and cooked herring (1105 mg per 100 grams).[32] Brains from mammals are also a good direct source. Beef brain, for example, contains approximately 855 mg of DHA per 100 grams in a serving.[33]
Discovery of algae-based DHA
In the early 1980s, NASA sponsored scientific research on a plant-based food source that could generate oxygen and nutrition on long-duration space flights. Certain species of marine algae produced rich nutrients, leading to the development of an algae-based, vegetable-like oil that contains two polyunsaturated fatty acids, DHA and arachidonic acid.[34]
Use as a food additive
DHA is widely used as a food supplement. It was first used primarily in infant formulas.[35] In 2019, the US Food and Drug Administration published qualified health claims for DHA.[36]
Some manufactured DHA is a vegetarian product extracted from algae, and it competes on the market with fish oil that contains DHA and other omega-3s such as EPA. Both fish oil and DHA are odorless and tasteless after processing as a food additive.[37]
Studies of vegetarians and vegans
Vegetarian diets typically contain limited amounts of DHA, and vegan diets typically contain no DHA.[38] In preliminary research, algae-based supplements increased DHA levels.[39] While there is little evidence of adverse health or cognitive effects due to DHA deficiency in adult vegetarians or vegans, breast milk levels remain a concern for supplying adequate DHA to the developing fetus.[38]
DHA and EPA in fish oils
Fish oil is widely sold in capsules containing a mixture of omega-3 fatty acids, including EPA and DHA. Oxidized fish oil in supplement capsules may contain lower levels of EPA and DHA.[40][41] Light, oxygen exposure, and heat can all contribute to oxidation of fish oil supplements.[40][41] Buying a quality product that is kept cold in storage and then keeping it in a refrigerator can help minimize oxidation.[42]
Hypothesized role in human evolution
An abundance of DHA in seafood has been suggested as being helpful in the development of a large brain,[43] though other researchers claim a terrestrial diet could also have provided the necessary DHA.[44]
References
- Guesnet P, Alessandri JM (2011). "Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) - Implications for dietary recommendations". Biochimie. 93 (1): 7–12. doi:10.1016/j.biochi.2010.05.005. PMID 20478353.
- "Archived copy". Archived from the original on 2013-07-07. Retrieved 2012-04-21.CS1 maint: archived copy as title (link)
- The omega end is the one furthest from the carboxyl group.
- Martek Biosciences Corporation (5 April 2007). "History of Martek". Archived from the original on February 5, 2007. Retrieved March 10, 2007.
- Martek Biosciences Corporation (29 July 2008). "Martek Products". Archived from the original on June 12, 2008. Retrieved July 29, 2008.
- Burdge, G. C.; Jones, A. E.; Wootton, S. A. (2002). "Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men". British Journal of Nutrition. 88 (4): 355–363. doi:10.1079/BJN2002662. PMID 12323085.
- Burdge, G. C.; Wootton, S. A. (2002). "Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women". British Journal of Nutrition. 88 (4): 411–20. doi:10.1079/BJN2002689. PMID 12323090.
- Malone, J. Patrick (2012). "The Systems Theory of Autistogenesis: Putting the Pieces Together". SAGE Open. 2 (2): 215824401244428. doi:10.1177/2158244012444281.
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL (2004). "Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects". The American Journal of Clinical Nutrition. 80 (5): 1167–74. doi:10.1093/ajcn/80.5.1167. PMID 15531662.
- Cederholm T, Salem N Jr, Palmblad J (2013). "ω-3 fatty acids in the prevention of cognitive decline in humans". Adv Nutr. 4 (6): 672–6. doi:10.3945/an.113.004556. PMC 3823515. PMID 24228198.
- Zimmer, Carl (September 17, 2015). "Inuit Study Adds Twist to Omega-3 Fatty Acids' Health Story". The New York Times. Retrieved October 11, 2015.
- O'Connor, Anahad (March 30, 2015). "Fish Oil Claims Not Supported by Research". The New York Times. Retrieved October 11, 2015.
- Grey, Andrew; Bolland, Mark (March 2014). "Clinical Trial Evidence and Use of Fish Oil Supplements". JAMA Internal Medicine. 174 (3): 460–462. doi:10.1001/jamainternmed.2013.12765. PMID 24352849.
- Kim, Hee-Yong; Huang, Bill X.; Spector, Arthur A. (2014). "Phosphatidylserine in the brain: Metabolism and function". Progress in Lipid Research. 56: 1–18. doi:10.1016/j.plipres.2014.06.002. ISSN 0163-7827. PMC 4258547. PMID 24992464.
- Singh, Meharban (March 2005). "Essential fatty acids, DHA and the human brain" (PDF). Indian Journal of Pediatrics. 72 (3): 239–242. doi:10.1007/BF02859265. PMID 15812120. Retrieved October 8, 2007.
- Spector, Arthur A.; Kim, Hee-Yong (2015). "Discovery of essential fatty acids". Journal of Lipid Research. 56 (1): 11–21. doi:10.1194/jlr.r055095. ISSN 0022-2275. PMC 4274059. PMID 25339684.
- Spector, Arthur A. (1999). "Essentiality of fatty acids". Lipids. 34: S1–S3. doi:10.1007/BF02562220. PMID 10419080.
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG (October 2005). "A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease". J Clin Invest. 115 (10): 2774–83. doi:10.1172/JCI25420. PMC 1199531. PMID 16151530.
- McNamara RK, Hahn CG, Jandacek R, et al. (2007). "Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder". Biol. Psychiatry. 62 (1): 17–24. doi:10.1016/j.biopsych.2006.08.026. PMID 17188654.
- McNamara, R. K.; Jandacek, R; Tso, P; Dwivedi, Y; Ren, X; Pandey, G. N. (2013). "Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease". Journal of Psychiatric Research. 47 (9): 1187–91. doi:10.1016/j.jpsychires.2013.05.007. PMC 3710518. PMID 23759469.
- De Caterina, R; Basta, G (June 2001). "n-3 Fatty acids and the inflammatory response – biological background". European Heart Journal Supplements. 3 (Supplement D): D42–D49. doi:10.1016/S1520-765X(01)90118-X.
- A Voss; M Reinhart; S Sankarappa; H Sprecher (October 1991). "The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase". The Journal of Biological Chemistry. 266 (30): 19995–20000. PMID 1834642. Retrieved January 2, 2011.
- Qiu, Xiao (2003-02-01). "Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): two distinct pathways". Prostaglandins, Leukotrienes and Essential Fatty Acids. 68 (2): 181–186. doi:10.1016/S0952-3278(02)00268-5. ISSN 0952-3278. PMID 12538082.
- Kuda, Ondrej (2017). "Bioactive metabolites of docosahexaenoic acid". Biochimie. 136: 12–20. doi:10.1016/j.biochi.2017.01.002. PMID 28087294.
- Westphal C, Konkel A, Schunck WH (Nov 2011). "CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease?". Prostaglandins & Other Lipid Mediators. 96 (1–4): 99–108. doi:10.1016/j.prostaglandins.2011.09.001. PMID 21945326.
- Harvard School Of Public Health. "Omega-3 Fatty Acids: An Essential Contribution". Retrieved 12 June 2015.
- Denomme J, Stark KD, Holub BJ (2005). "Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations". The Journal of Nutrition. 135 (2): 206–11. doi:10.1093/jn/135.2.206. PMID 15671214.
- Hüppi PS (March 2008). "Nutrition for the brain: commentary on the article by Isaacs et al. on page 308" (PDF). Pediatric Research. 63 (3): 229–31. doi:10.1203/pdr.0b013e318168c6d1. PMID 18287959.
- Harris WS, Baack ML (January 2015). "Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity". Journal of Perinatology. 35 (1): 1–7. doi:10.1038/jp.2014.195. PMC 4281288. PMID 25357095.
- SanGiovanni JP, Chew EY (January 2005). "The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina". Progress in Retinal and Eye Research. 24 (1): 87–138. doi:10.1016/j.preteyeres.2004.06.002. PMID 15555528.
- Schwartz, Stephen G.; Wang, Xue; Chavis, Pamela; Kuriyan, Ajay E.; Abariga, Samuel A. (18 June 2020). "Vitamin A and fish oils for preventing the progression of retinitis pigmentosa". The Cochrane Database of Systematic Reviews. 6: CD008428. doi:10.1002/14651858.CD008428.pub3. ISSN 1469-493X. PMID 32573764.
- "EPA and DHA Content of Fish Species. Appendix G2". US Department of Agriculture. 2005. Retrieved 15 September 2013.
- "Beef, variety meats and by-products, brain, cooked, simmered". Retrieved 2011-10-27.
- Jones, John. "Nutritional Products from Space Research". May 1st, 2001. NASA.
- "FDA: Why is there interest in adding DHA and ARA to infant formulas?". US Food & Drug Administration. Retrieved 1 July 2002.
- "FDA Announces New Qualified Health Claims for EPA and DHA Omega-3 Consumption and the Risk of Hypertension and Coronary Heart Disease". US Food and Drug Administration. 19 June 2019. Retrieved 30 August 2019.
- Rivlin, Gary (2007-01-14). "Magical or Overrated? A Food Additive in a Swirl". The New York Times. Retrieved 2007-01-15.
- Sanders, T. A. (2009). "DHA status of vegetarians". Prostaglandins, Leukotrienes and Essential Fatty Acids. 81 (2–3): 137–41. doi:10.1016/j.plefa.2009.05.013. PMID 19500961.
- Lane, K; Derbyshire, E; Li, W; Brennan, C (2014). "Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature". Critical Reviews in Food Science and Nutrition. 54 (5): 572–9. doi:10.1080/10408398.2011.596292. PMID 24261532.
- Albert, Benjamin B (21 January 2015). "Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA release". Scientific Reports. 5: 7928. doi:10.1038/srep07928. PMC 4300506. PMID 25604397.
- Albert, Benjamin B; Cameron-Smith, David; Hofman, Paul L.; Cutfield, Wayne S. (2013). "Oxidation of Marine Omega-3 Supplements and Human Health". BioMed Research International. 2013: 464921. doi:10.1155/2013/464921. PMC 3657456. PMID 23738326.
- Zargar, Atanaz; Ito, Matthew K. (1 August 2011). "Long chain omega-3 dietary supplements: a review of the National Library of Medicine Herbal Supplement Database". Metabolic Syndrome and Related Disorders. 9 (4): 255–271. doi:10.1089/met.2011.0004. ISSN 1557-8518. PMID 21787228.
- Crawford, M; et al. (2000). "Evidence for the unique function of docosahexaenoic acid (DHA) during the evolution of the modern hominid brain". Lipids. 34 (S1): S39–S47. doi:10.1007/BF02562227. PMID 10419087.
- Carlson BA, Kingston JD (2007). "Docosahexaenoic acid biosynthesis and dietary contingency: Encephalization without aquatic constraint". Am. J. Hum. Biol. 19 (4): 585–8. doi:10.1002/ajhb.20683. PMID 17546613.