Biometal (biology)
Biometals are metals normally present, in small but important and measurable amounts, in biology, biochemistry, and medicine. The metals copper, zinc, iron, and manganese are examples of metals that are essential for the normal functioning of most plants and the bodies of most animals, such as the human body. A few (calcium, potassium, sodium) are present in relatively larger amounts, whereas most others are trace metals, present in smaller but important amounts (the image shows the percentages for humans). Approximately 2/3 of the existing periodic table is composed of metals with varying properties,[1] accounting for the diverse ways in which metals (usually in ionic form) have been utilized in nature and medicine.
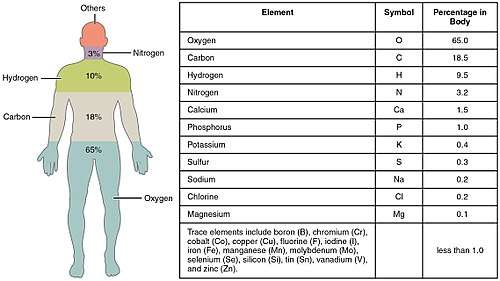
Naturally occurring biometals
Metal ions are essential to the function of many proteins present in living organisms, such as metalloproteins and enzymes that require metal ions as cofactors.[2] Processes including oxygen transport and DNA replication are carried out using enzymes such as DNA polymerase, which in humans requires magnesium and zinc to function properly.[3] Other biomolecules also contain metal ions in their structure, such as iodine in human thyroid hormones.[4]
Biometals in medicine
Metal ions and metallic compounds are often used in medical treatments and diagnoses.[5] Compounds containing metal ions can be used as medicine, such as lithium compounds and auranofin.[6][7] Metal compounds and ions can also produce harmful effects on the body due to the toxicity of several types of metals.[8] For example, arsenic works as a potent poison due to its effects as an enzyme inhibitor, disrupting ATP production.[9]
References
- http://rna.cshl.edu/content/free/chapters/12_rna_world_2nd.pdf
- Banci, Lucia, ed. (2013). Metallomics and the Cell. Series editors Sigel, Astrid; Sigel, Helmut; Sigel, Roland K.O. Springer. ISBN 978-94-007-5560-4. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- Aggett, PJ (1985). "Physiology and metabolism of essential trace elements: an outline". Clin Endocrinol Metab. 14 (3): 513–43. doi:10.1016/S0300-595X(85)80005-0. PMID 3905079.
- Cavalieri, RR (1997). "Iodine metabolism and thyroid physiology: current concepts". Thyroid. 7 (2): 177–81. doi:10.1089/thy.1997.7.177. PMID 9133680.
- http://authors.library.caltech.edu/25052/10/BioinCh_chapter9.pdf Stephen J. Lippard, Department of Chemistry, Massachusetts Institute of Technology. Accessed 26 July 2014.
- https://www.nlm.nih.gov/medlineplus/druginfo/meds/a681039.html U.S. National Library of Medicine, Lithium. Drug information provided by AHFS Consumer Medication Information, 2014.
- Kean, W. F.; Hart, L.; Buchanan, W. W. (1997). "Auranofin". British Journal of Rheumatology. 36 (5): 560–572. doi:10.1093/rheumatology/36.5.560. PMID 9189058.
- http://authors.library.caltech.edu/25052/10/BioinCh_chapter9.pdf Stephen J. Lippard, Department of Chemistry, Massachusetts Institute of Technology. Accessed 26 July 2014.
- Singh, AP; Goel, RK; Kaur, T (2011). "Mechanisms pertaining to arsenic toxicity". Toxicology International. 18 (2): 87–93. doi:10.4103/0971-6580.84258. PMC 3183630. PMID 21976811.