Selumetinib
Selumetinib (INN),[1] sold under the brand name Koselugo, is a medication for the treatment of children, two years of age and older, with neurofibromatosis type I (NF1), a genetic disorder of the nervous system causing tumors to grow on nerves.[2] It is taken by mouth twice per day on an empty stomach.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Koselugo |
| Other names | AZD6244, ARRY-142886 |
| AHFS/Drugs.com | Koselugo |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Protein kinase inhibitor |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.311 |
| Chemical and physical data | |
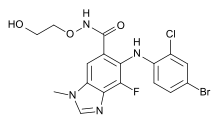
| Formula | C17H15BrClFN4O3 |
| Molar mass | 457.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects are vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain (pain in the body affecting bones, muscles, ligaments, tendons and nerves), fever, acneiform rash (acne), stomatitis (inflammation of the mouth and lips), headache, paronychia (infection in the skin that surrounds a toenail or fingernail) and pruritus (itching).[2]
Mechanism of action
The gene BRAF is part of the MAPK/ERK pathway, a chain of proteins in cells that communicates input from growth factors. Activating mutations in the BRAF gene, primarily V600E (meaning that the amino acid valine in position 600 is replaced by glutamic acid), are associated with lower survival rates in patients with papillary thyroid cancer. Another type of mutation that leads to undue activation of this pathway occurs in the gene KRAS and is found in NSCLC. A possibility of reducing the activity of the MAPK/ERK pathway is to block the enzyme MAPK kinase (MEK), immediately downstream of BRAF, with the drug selumetinib. More specifically, selumetinib blocks the subtypes MEK1 and MEK2 of this enzyme.[4]
Adverse effects
Selumetinib can also cause serious side effects including heart failure (manifested as ejection fraction decrease, or when the muscle of the left ventricle of the heart is not pumping as well as normal) and eye toxicity (acute and chronic damage to the eye) including retinal vein occlusion, retinal pigment epithelial detachment and impaired vision.[2] Cardiac and ophthalmic assessments should be performed prior to initiating selumetinib and at regular intervals during treatment. Selumetinib can also cause increased creatinine phosphokinase (CPK).[2] CPK is an enzyme found in the heart, brain and skeletal muscles.[2] When muscle tissue is damaged, CPK leaks into a person's blood.[2] CPK elevation in one receiving selumetinib should prompt an evaluation for rhabdomyolysis (breakdown of skeletal muscle due to direct or indirect muscle injury).[2] Selumetinib should be withheld, dosage reduced or dosage permanently discontinued based on the severity of adverse reactions.[2] Further, selumetinib contains vitamin E, and users are at an increased risk of bleeding if their daily intake of vitamin E exceeds the recommended or safe limits.[2]
Pregnancy
Based on findings from animal studies, selumetinib may cause harm to a newborn baby when administered to a pregnant woman.[2] The FDA advises health care professionals to tell females of reproductive age, and males with female partners of reproductive potential, to use effective contraception during treatment with selumetinib, and for one week after the last dose.[2]
Possible uses
Treatment of neurofibromas in those with neurofibromatosis.
In addition to thyroid cancer, BRAF-activating mutations are prevalent in melanoma (up to 59%), colorectal cancer (5–22%), serous ovarian cancer (up to 30%), and several other tumor types.[5]
Selumetinib has also been shown to inhibit growth of GNAQ mutated uveal melanoma cell lines.[6] Furthermore, preliminary results suggest that selumetinib treatment of uveal melanoma patients can result in tumor shrinkage as the consequence of sustained inhibition of ERK phosphorylation.[7]
KRAS mutations appear in 20 to 30% of NSCLC cases and about 40% of colorectal cancer.[4]
A Phase II clinical trial about selumetinib in NSCLC was completed in September 2011;[8] one about cancers with BRAF mutations is ongoing as of June 2012.[9]
In July 2015, selumetinib failed a Phase III trial testing whether the drug significantly prolonged the survival of patients in a study on melanoma originating in the eye. In the 152-patient trial, a combination of selumetinib and dacarbazine failed to improve progression-free survival compared with just the old drug alone.[10][11]
As of March 2016, there were other phase III trials registered for thyroid cancer,[12] and KRAS Positive NSCLC.[13] The combination of selumetinib to chemotherapy improved median progression-free survival}}</ref> in a trial of 510 patients with advanced KRAS-mutant non-small cell lung cancer (NSCLC) just for one month, which was statistically not significant.[14]
In November 2018, investigators working with nasal polyp tissue in vitro demonstrated a synergistic effect of down regulating expression of p-MEK1 and p-ERK1 when it was administered with erythromycin.
History
Selumetinib was discovered by Array BioPharma and was licensed to AstraZeneca. It has been investigated for the treatment of various types of cancer, such as non-small cell lung cancer (NSCLC) and thyroid cancer.[15][16]
In April 2020, selumetinib was approved in the United States for the treatment of children with neurofibromatosis type 1 (NF1).[2][17][18] Selumetinib is approved specifically for children who have symptomatic, inoperable plexiform neurofibromas (PN), which are tumors involving the nerve sheaths (coating around nerve fibers) and can grow anywhere in the body, including the face, extremities, areas around the spine and deep in the body where they may affect organs.[2] Selumetinib is a kinase inhibitor, meaning it functions by blocking a key enzyme, which results in helping to stop the tumor cells from growing.[2]
NF1 is a rare, progressive condition caused by a mutation or flaw in a particular gene.[2] NF1 is usually diagnosed in early childhood and appears in an estimated one out of every 3,000 infants.[2] It is characterized by changes in skin coloring (pigmentation), neurologic and skeletal impairments and risk for development of benign and malignant tumors throughout life.[2] Between 30% and 50% of children born with NF1 develop one or more PNs.[2]
Selumetinib is the first drug approved by the US Food and Drug Administration (FDA) to treat this debilitating, progressive and often disfiguring rare disease that typically begins early in life.[2]
The FDA approved selumetinib based on a clinical trial (NCT01362803) conducted by the National Cancer Institute of children who had NF1 and inoperable PN (defined as a PN that could not be completely removed without risk for substantial morbidity to the subject).[2][18] The efficacy results were from 50 of the subjects who received the recommended dose and had routine evaluations of changes in tumor size and tumor-related morbidities during the trial.[2] Subjects received selumetinib 25 mg/m2 orally twice a day until disease progression or until they experienced unacceptable adverse reactions.[2][18] The clinical trial measured the overall response rate (ORR), defined as the percentage of subjects with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3–6 months.[2] The ORR was 66% and all subjects had a partial response, meaning that no subjects had complete disappearance of the tumor.[2] Of these subjects, 82% had a response lasting 12 months or longer.[2] The trial was conducted at four sites in the United States.[18]
Other clinical outcomes for subjects during selumetinib treatment including changes in PN-related disfigurement, symptoms and functional impairments.[2] Although the sample sizes of subjects assessed for each PN-related morbidity (such as disfigurement, pain, strength and mobility problems, airway compression, visual impairment and bladder or bowel dysfunction) were small, there appeared to be a trend of improvement in PN-related symptoms or functional deficits during treatment.[2]
The FDA granted the application for selumetinib priority review, breakthrough therapy, and orphan drug designations.[2] It was granted a rare pediatric disease designation for the treatment of pediatric NF1 along with a rare pediatric disease priority review voucher.[2] The FDA granted approval of Koselugo to AstraZeneca Pharmaceuticals LP.[2]
References
- World Health Organization (2009). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 62". WHO Drug Information. 23 (3): 261. hdl:10665/74420.
- "FDA Approves First Therapy for Children with Debilitating and Disfiguring Rare Disease". U.S. Food and Drug Administration (FDA) (Press release). 10 April 2020. Retrieved 10 April 2020.

- "Koselugo- selumetinib capsule". DailyMed. 10 April 2020. Retrieved 18 April 2020.
- Troiani T, Vecchione L, Martinelli E, Capasso A, Costantino S, Ciuffreda LP, et al. (May 2012). "Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells". British Journal of Cancer. 106 (10): 1648–59. doi:10.1038/bjc.2012.129. PMC 3349172. PMID 22569000.
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. (June 2002). "Mutations of the BRAF gene in human cancer" (PDF). Nature. 417 (6892): 949–54. doi:10.1038/nature00766. PMID 12068308.
- Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK (July 2012). "Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance". Clinical Cancer Research. 18 (13): 3552–61. doi:10.1158/1078-0432.CCR-11-3086. PMC 3433236. PMID 22550165.
- "Pharmacodynamic activity of selumetinib to predict radiographic response in advanced uveal melanoma". 2012.
- "AZD6244 in Combination With Docetaxel Versus Docetaxel Alone in KRAS Mutation Positive NSCLC Patients". ClinicalTrials.gov. 30 April 2009. Retrieved 10 April 2020.
- "Selumetinib in Cancers With BRAF Mutations". ClinicalTrials.gov. 27 April 2009. Retrieved 10 April 2020.
- "AstraZeneca provides update on selumetinib in uveal melanoma". AstraZeneca (Press release). 22 July 2015. Retrieved 10 April 2020.
- "AstraZeneca's once-lauded drug flunks a Phase III eye cancer trial". FierceBiotech.
- "Comparing Complete Remission After Treatment With Selumetinib/Placebo in Patient With Differentiated Thyroid Cancer (ASTRA)". ClinicalTrials.gov. 30 April 2013. Retrieved 10 April 2020.
- "Assess Efficacy & Safety of Selumetinib in Combination With Docetaxel in Patients Receiving 2nd Line Treatment for v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Positive NSCLC (SELECT-1)". ClinicalTrials.gov. 2 September 2013. Retrieved 10 April 2020.
- Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. (May 2017). "Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial". JAMA. 317 (18): 1844–53. doi:10.1001/jama.2017.3438. PMC 5815037. PMID 28492898.
- "Array BioPharma strikes rights deal with Japanese firm worth up to $76M-plus". BizWest. 31 March 2016. Retrieved 12 June 2018.
- Casaluce F, Sgambato A, Maione P, Sacco PC, Santabarbara G, Gridelli C (August 2017). "Selumetinib for the treatment of non-small cell lung cancer". Expert Opinion on Investigational Drugs. 26 (8): 973–84. doi:10.1080/13543784.2017.1351543. PMID 28675058.
- "Selumetinib: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 10 April 2020.
- "Drug Trials Snapshots: Koselugo". U.S. Food and Drug Administration (FDA). 10 April 2020. Retrieved 18 April 2020.

Further reading
- Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. (February 2013). "Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer". The New England Journal of Medicine. 368 (7): 623–32. doi:10.1056/NEJMoa1209288. PMC 3615415. PMID 23406027.
External links
- "Selumetinib". Drug Information Portal. U.S. National Library of Medicine.
- "Selumetinib". National Cancer Institute.
- Clinical trial number NCT01362803 for "AZD6244 Hydrogen Sulfate for Children With Nervous System Tumors" at ClinicalTrials.gov