Ibrutinib
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that binds permanently to a protein, Bruton's tyrosine kinase (BTK), that is important in B cells. It is used to treat B cell cancers like mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Imbruvica, Ibrutix |
| Other names | PCI-32765, CRA-032765 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614007 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 97.3% |
| Metabolism | Hepatic (CYP3A & CYP2D6) |
| Elimination half-life | 4–6 hours |
| Excretion | Feces (80%), urine (10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.232.543 |
| Chemical and physical data | |
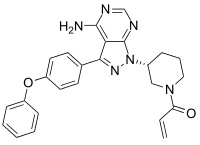
| Formula | C25H24N6O2 |
| Molar mass | 440.4971 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
Ibrutinib is used to treat chronic lymphocytic leukemia (CLL), Waldenström's macroglobulinemia, and as a second-line treatment for mantle cell lymphoma, marginal zone lymphoma, and chronic graft vs host disease.[4][3]
In the United States ibrutinib is indicated for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy, adults with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) with or without 17p deletion, adults with Waldenström's macroglobulinemia (WM), adults with marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy, adults with chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy.[3][5][6][7][8]
It is a first line treatment in those with CLL who require treatment and are newly diagnosed.[9] It may also be used in CLL that relapses.[9]
Resistance
Both primary (inherent) and secondary (acquired) resistance has been reported in various lymphomas, including CLL and MCL.[10] Resistance may arise due to mutations that impair the affinity of ibrutinib for BTK, or due to alterations in pathways downstream of BTK and may confer BCR signaling independence in resistant clones.
Adverse effects
Very common (>10% frequency) adverse effects include pneumonia, upper respiratory tract infection, sinusitis, skin infection, low neutrophil count, low platelet counts, headache, bleeding, bruising, diarrhea, vomiting, inflammation of mouth and lips, nausea, constipation, rash, joint pain, muscle spasms, musculoskeletal pain, fever, and edema.[4]
Common (1–10% frequency) adverse effects include sepsis, urinary tract infection, non-melanoma skin cancer (basal-cell carcinoma, squamous cell carcinoma), low leukocyte count, low lymphocyte count, interstitial lung disease, tumor lysis syndrome, high uric acid levels, dizziness, blurred vision, atrial fibrillation, subdural hematoma, nosebleeds, small bruises from broken blood vessels, high blood pressure, hives, and skin redness or blushing.[4]
Pharmacology
Ibrutinib oral bioavailability is 3.9% in a fasting state, 8.4% in a fed state, and 15.9% after consumption of grapefruit juice.[11]
Mechanism
Ibrutinib has been reported to reduce chronic lymphocytic leukemia cell chemotaxis towards the chemokines CXCL12 and CXCL13, and inhibit cellular adhesion following stimulation at the B-cell receptor (BCR).[12][13] Additionally, ibrutinib down-modulates the expression of CD20 (target of rituximab/ofatumumab) by targeting the CXCR4/SDF1 axis.[14] Together, these data are consistent with a mechanistic model whereby ibrutinib blocks BCR signaling, which drives cells into apoptosis and/or disrupts cell migration and adherence to protective tumour microenvironments.
In preclinical studies on chronic lymphocytic leukemia (CLL) cells, ibrutinib has been reported to promote apoptosis, inhibit proliferation, and also prevent CLL cells from responding to survival stimuli provided by the microenvironment.[14] This also leads to a reduction of MCL1 levels (anti-apoptotic protein) in malignant B cells.[14] Treatment of activated CLL cells with ibrutinib resulted in inhibition of BTK tyrosine phosphorylation and also effectively abrogated downstream survival pathways activated by this kinase including ERK1/2, PI3K, and NF-κB. Additionally, ibrutinib inhibited proliferation of CLL cells in vitro, effectively blocking survival signals provided externally to CLL cells from the microenvironment including soluble factors (CD40L, BAFF, IL-6, IL-4, and TNF-α), fibronectin engagement and stromal cell contact.
In early clinical studies, the activity of ibrutinib has been described to include a rapid reduction in lymphadenopathy accompanied by a transient lymphocytosis, suggesting that the drug might have direct effects on cell homing or migration to factors in tissue microenvironments.[15]
History
Ibrutinib was created by scientists at Celera Genomics as a tool compound for studying BTK function; it covalently binds its target which is ideal for a reagent but generally not considered ideal for drugs.[16]
In 2006, in the course of acquiring an HDAC-focused program from Celera after its own initial discovery program had failed, Pharmacyclics also picked up Celera's small molecule BTK inhibitor discovery program for $2M in cash and $1M in stock and named the tool compound PCI-32765.[16][17] In 2011 after the drug had completed Phase II trials, Johnson & Johnson and Pharmacyclics agreed to co-develop the drug, and J&J paid Pharmacyclics $150 million upfront and $825 million in milestones.[18] Pharmacyclics was acquired by AbbVie in May 2015, and Abbvie projected global sales of US$1 billion in 2016 and $5 billion in 2020.[19]
It was approved by the US Food and Drug Administration (FDA) on November 13, 2013, for the treatment of mantle cell lymphoma.[5] On February 12, 2014, the FDA expanded the approved use of ibrutinib to chronic lymphocytic leukemia (CLL).[20][21] It was approved for Waldenström's macroglobulinemia in 2015.[6][22]
In March 2015, Pharmacyclics and AbbVie agreed that Abbvie would acquire Pharmacyclics for $21 billion;[23] the deal was completed that May.[24]
In March 2016, a new indication for ibrutinib was approved in the United States for patients with chronic lymphocytic leukemia (CLL).[25]
In May 2016, a new indication for ibrutinib was approved in the United States for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).[26]
In January 2017, a new indication for ibrutinib was approved in the United States for the treatment of adults with relapsed/refractory (R/R) marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy.[27]
In August 2017, the FDA approved a new indication for ibrutinib to treat graft-versus-host disease. It was the first drug approved by the FDA for this condition.[7][8][28]
In February 2018, a tablet formulation of ibrutinib was approved for use in the United States.[29]
In August 2018, ibrutinib in combination with rituximab was approved in the United States for the treatment of adults with Waldenström's macroglobulinemia (WM), a rare and incurable type of non-Hodgkin's lymphoma (NHL).[30]
In January 2019, ibrutinib in combination with obinutuzumab was approved for the treatment of adults with previously untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).[31]
In April 2020, the FDA expanded the indication of ibrutinib to include its combination with rituximab for the initial treatment of adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).[32] Approval was based on the E1912 trial (NCT02048813), a 2:1 randomized, multicenter, open-label, actively controlled trial of ibrutinib with rituximab compared to fludarabine, cyclophosphamide, and rituximab (FCR) in 529 adult subjects 70 years or younger with previously untreated CLL or SLL requiring systemic therapy.[32]
Cost
The typical cost of ibrutinib in the United States is about $148,000 a year. Preliminary PK/PD focused research found that people could potentially be put on lower and less expensive regimen of ibrutinib without losing efficiency; however, no data showing efficiency of lower doses has been published.
Janssen Pharmaceutica and Pharmacyclics introduced a new single dose tablet formulation with a flat pricing structure in the first half of 2018 and discontinue the capsule formulation. This caused an outcry as it was perceived to triple in the cost of the drug to the average patient.[33] Patients receiving the FDA approved and recommended doses would have seen either no price change or a price decrease with the tablet pricing structure.
Janssen Pharmaceutica and Pharmacyclics have since reversed the decision to discontinue the capsule formulation with the drug currently available in both capsule and tablet forms.[34]
Ibrutinib was added to the Australian Pharmaceutical Benefits Scheme in 2018.[35]
Generic ibrutinib was added to the Indian Pharmaceutical Benefits Scheme in 2020.[36]
Brand names
In Bangladesh it is available under the brandname Ibrutix by Beacon Pharmaceuticals.
References
- "Ibrutinib (Imbruvica) Use During Pregnancy". Drugs.com. 3 December 2019. Retrieved 28 March 2020.
- "Imbruvica 140 mg Film-Coated Tablets - Summary of Product Characteristics (SmPC)". (emc). 16 January 2020. Retrieved 28 March 2020.
- "Imbruvica- ibrutinib capsule Imbruvica- ibrutinib tablet, film coated". DailyMed. 8 April 2020. Retrieved 21 April 2020.
- "UK Ibrutiniib label". UK Electronic Medicines Compendium. 25 August 2016.
- "FDA approves Imbruvica for rare blood cancer". U.S. Food and Drug Administration (FDA) (Press release). 13 November 2013.

- "FDA expands approved use of Imbruvica for rare form of non-Hodgkin lymphoma" (Press release). U.S. Food and Drug Administration (FDA). January 29, 2015.

- "FDA approves treatment for chronic graft versus host disease" (Press release). U.S. Food and Drug Administration (FDA). 2 August 2017. Retrieved 28 March 2020.

- "FDA expands ibrutinib indications to chronic GVHD". U.S. Food and Drug Administration (FDA). 2 August 2017. Retrieved 21 April 2020.
- "Chronic Lymphocytic Leukemia Treatment". National Cancer Institute. 1 January 1980. Retrieved 19 February 2019.
- Kaur V (2017). "Ibrutinib in CLL: a focus on adverse events, resistance and novel approaches beyond ibrutinib". Ann Hematol. doi:10.1007/s00277-017-2973-2. PMID 28342031.
- deVries R (2016). "Stable isotope-labelled intravenous microdose for absolute bioavailability and effect of grapefruit juice on ibrutinib in healthy adults". Br J Clin Pharmacol. 81 (2): 235–45. doi:10.1111/bcp.12787. PMC 4833163. PMID 26382728.
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O'Brien S, Chiorazzi N, Burger JA (Feb 2012). "The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo". Blood. 119 (5): 1182–1189. doi:10.1182/blood-2011-10-386417. PMC 4916557. PMID 22180443.
- de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M (Mar 2012). "The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia". Blood. 119 (11): 2590–2594. doi:10.1182/blood-2011-11-390989. PMID 22279054.
- Pavlasova, G; et al. (22 September 2016). "Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis". Blood. 128 (12): 1609–13. doi:10.1182/blood-2016-04-709519. PMC 5291297. PMID 27480113.
- Brown JR (2013). "Ibrutinib (PCI-32765), the first BTK (Bruton's tyrosine kinase) inhibitor in clinical trials". Curr Hematol Malig Rep. 8 (1): 1–6. doi:10.1007/s11899-012-0147-9. PMC 3584329. PMID 23296407.
- Shaywitz, David (April 5, 2013). "The Wild Story Behind A Promising Experimental Cancer Drug". Forbes.
- Langreth, Robert; Coffey, Brendan (26 February 2015). "Cancer Drug Once Bought for $7 Million May Now Fetch $18 Billion". Bloomberg.com.
- Sheridan, C (7 March 2012). "Companies in rapid pursuit of Btk immunokinase". Nature Biotechnology. 30 (3): 199–200. doi:10.1038/nbt0312-199. PMID 22398595.
- Walker, Joseph (1 January 2016). "Patients Struggle With High Drug Prices: Out-of-pocket costs for pricey new drugs leave even some insured and relatively affluent patients with hard choices on how to afford them". The Wall Street Journal. Retrieved 31 January 2019.
- "Imbruvica (ibrutinib) Capsules". U.S. Food and Drug Administration (FDA). 8 April 2015. Retrieved 21 April 2020.
- Azvolinsky, Anna. "FDA Approves Ibrutinib for Chronic Lymphocytic Leukemia". Cancer Network. Retrieved 14 February 2014.
- "IMBRUVICA (ibrutinib) Now Approved to Treat Waldenstrom's Macroglobulinemia in Europe". AbbVie. 10 July 2015. Retrieved 21 April 2020.
- Rockoff, Jonathan D.; Loftus, Peter (5 March 2015). "AbbVie to Buy Pharmacyclics in $21 Billion Deal". The Wall Street Journal.
- Sachdev, Ameet (May 26, 2015). "AbbVie closes $21 billion deal for Pharmacyclics". Chicago Tribune.
- "IMBRUVICA (ibrutinib) Approved by U.S. FDA for the First-line Treatment of Chronic Lymphocytic Leukemia". AbbVie (Press release). 4 March 2016. Retrieved 21 April 2020.
- "U.S. FDA Expands IMBRUVICA (ibrutinib) Label to Include Overall Survival Data in Previously Untreated Chronic Lymphocytic Leukemia (CLL) and New Indication for Small Lymphocytic Lymphoma (SLL) Patients". AbbVie (Press release). 9 May 2016. Retrieved 21 April 2020.
- "U.S. FDA Approves IMBRUVICA (ibrutinib) as First Treatment Specifically Indicated for Relapsed/Refractory Marginal Zone Lymphoma (MZL) - a Rare Type of Non-Hodgkin's Lymphoma". AbbVie (Press release). 19 January 2017. Retrieved 21 April 2020.
- "U.S. FDA Approves IMBRUVICA (ibrutinib) as First Approved Treatment Specifically for Adults with Chronic Graft-Versus-Host-Disease (cGVHD) -- A Serious, Potentially Life-Threatening Condition -- After Failure of One or More Lines of Systemic Therapy". AbbVie (Press release). 2 August 2017. Retrieved 21 April 2020.
- "Drug Approval Package: Imbruvica (ibrutinib)". U.S. Food and Drug Administration (FDA). 26 October 2018. Retrieved 22 April 2020.
- "AbbVie Announces IMBRUVICA (ibrutinib) Plus Rituximab Approval by U.S. FDA as First Chemotherapy-Free Combination Treatment in Adults with Waldenström's Macroglobulinemia, a Rare Type of Blood Cancer". AbbVie (Press release). 27 August 2018. Retrieved 21 April 2020.
- "AbbVie Announces U.S. FDA Approval of IMBRUVICA (ibrutinib) Plus Obinutuzumab (GAZYVA) - First Chemotherapy-Free, Anti-CD20 Combination Regimen Approved for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) in Previously Untreated Patients". AbbVie (Press release). 28 January 2019. Retrieved 21 April 2020.
- "FDA approves ibrutinib plus rituximab for chronic lymphocytic leukemia". U.S. Food and Drug Administration (FDA). 21 April 2020. Retrieved 21 April 2020.

- Johnson, Carolyn Y. (2018-04-18). "Science hinted that cancer patients could take less of a $148,000-a-year drug. Its maker tripled the price of a pill". The Washington Post. Retrieved 2018-04-19.
- Johnson, Carolyn Y. (2018-05-15). "After outcry, drugmakers decide not to triple the price of a cancer pill". The Washington Post. Retrieved 2018-06-13.
- "MIL-OSI Australia: $250 million investment in life changing cancer medicines – ForeignAffairs.co.nz". Foreignaffairs.co.nz. 16 July 2018. Retrieved 20 July 2018.
- "Cost-effectiveness Ibrutinib [Imbruvica] In India, USA, UK, And Australia – Medixocentre.com". Medixocentre.com. 10 Feb 2020. Retrieved 15 Feb 2020.
External links
- "Ibrutinib". Drug Information Portal. U.S. National Library of Medicine.
- Ibrutinib, National Cancer Institute Drug Dictionary