Plectin
Plectin is a giant protein found in nearly all mammalian cells which acts as a link between the three main components of the cytoskeleton: actin microfilaments, microtubules and intermediate filaments.[5] In addition plectin links the cytoskeleton to junctions found in the plasma membrane that structurally connect different cells. By holding these different networks together plectin plays an important role in maintaining the mechanical integrity and viscoelastic properties of tissues.[6]
Structure
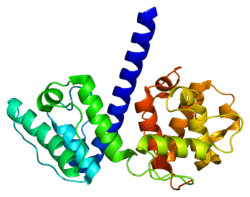
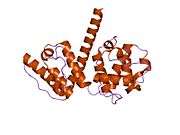
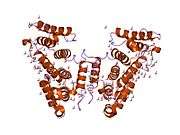
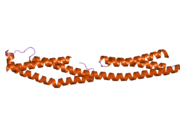
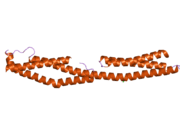
Plectin can exist in cells as several alternatively-spliced isoforms, all around 500 kDa and >4000 amino acids.[7][8] The structure of plectin is thought to be a dimer consisting of a central coiled coil of alpha helices connecting two large globular domains (one at each terminus). These globular domains are responsible for connecting plectin to its various cytoskeletal targets. The carboxy-terminal domain is made of 6 highly homologous repeating regions. The subdomain between regions five and six of this domain is known to connect to the intermediate filaments cytokeratin and vimentin. At the opposite end of the protein, in the N-terminal domain, a region has been defined as responsible for binding to actin.[9] In 2004, the exact crystal structure of this actin-binding domain (ABD) was determined in mice and shown to be composed of two calponin homology (CH) domains.[10] Plectin is expressed in nearly all mammalian tissues. In cardiac muscle and skeletal muscle, plectin is localized to specialized entities known as Z-discs.[11] Plectin binds several proteins, including vinculin, DES,[12] actin.,[6][13] fodrin,[6][13] microtubule-associating proteins,[6][13] nuclear laminin B.,[6][13] SPTAN1,[14][15] vimentin[14][15][16] and ITGB4.[6][13]
Function
Studies employing a plectin knockout mouse have shed light on the functions of plectin. Pups died 2–3 days after birth, and these mice exhibited marked skin abnormalities, including degeneration of keratinocytes. Skeletal and cardiac muscle tissues were also significantly affected. Cardiac intercalated discs were disintegrated and sarcomeres were irregularly shapen, and intracellular accumulation of aberrant isolated myofibrillar bundles and Z-disc components was also observed. Expression of vinculin in muscle cells was strikingly down-regulated.[17] Through the use of gold-immunoelectron microscopy, immunoblotting and immunofluorescence experiments plectin has been found to associate with all three major components of the cytoskeleton. In muscle, plectin binds to the periphery of Z-discs,[12] and along with the intermediate filament protein desmin, may form lateral linkages among neighboring Z-discs. This interaction between plectin and desmin intermediate filaments also appears to facilitate the close association of myofibrils and mitochondria, both at Z-discs and along the remainder the sarcomere.[18] Plectin also functions to link cytoskeleton to intercellular junctions, such as desmosomes and hemidesmosomes, which link intermediate filament networks between cells. Plectin has been revealed to localize to the desmosomes and in vitro studies have shown that it can form bridges between the desmosome protein, desmoplakin and intermediate filaments.[19] In hemidesmosomes plectin has been shown to interact with the integrin β4 subunits of the hemidesmosome plaque and function in a clamp-like manner to link the intermediate filament cytokeratin to the junction.[20]
Clinical significance
Mutations in PLEC have been associated with epidermolysis bullosa simplex with muscular dystrophy. A missense variant of PLEC has been recently proposed as a cause of atrial fibrillation in some populations.[21] Isolated left ventricular non-compaction accompanying epidermolysis bullosa simplex with muscular dystrophy was also noted.[22] Plectin has been proposed as a biomarker for pancreatic cancer.[23][24] Although normally a cytoplasmic protein, plectin is expressed on the cell membrane in pancreatic ductal adenocarcinoma (PDAC) and can therefore be used to target PDAC cells.[23]
See also
References
- GRCh38: Ensembl release 89: ENSG00000178209 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022565 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Svitkina TM, Verkhovsky AB, Borisy GG (Nov 1996). "Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton". The Journal of Cell Biology. 135 (4): 991–1007. doi:10.1083/jcb.135.4.991. PMC 2133373. PMID 8922382.
- Wiche G (Sep 1998). "Role of plectin in cytoskeleton organization and dynamics" (abstract). Journal of Cell Science. 111 (17): 2477–86. PMID 9701547.
- "Archived copy". Archived from the original on 2016-03-05. Retrieved 2015-04-13.CS1 maint: archived copy as title (link)
- Zong, N. C.; Li, H; Li, H; Lam, M. P.; Jimenez, R. C.; Kim, C. S.; Deng, N; Kim, A. K.; Choi, J. H.; Zelaya, I; Liem, D; Meyer, D; Odeberg, J; Fang, C; Lu, H. J.; Xu, T; Weiss, J; Duan, H; Uhlen, M; Yates Jr, 3rd; Apweiler, R; Ge, J; Hermjakob, H; Ping, P (2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- Winter L, Wiche G (Jan 2013). "The many faces of plectin and plectinopathies: pathology and mechanisms". Acta Neuropathologica. 125 (1): 77–93. doi:10.1007/s00401-012-1026-0. PMID 22864774.
- Sevcík J, Urbániková L, Kost'an J, Janda L, Wiche G (May 2004). "Actin-binding domain of mouse plectin. Crystal structure and binding to vimentin". European Journal of Biochemistry / FEBS. 271 (10): 1873–84. doi:10.1111/j.1432-1033.2004.04095.x. PMID 15128297.
- Zernig G, Wiche G (Jul 1985). "Morphological integrity of single adult cardiac myocytes isolated by collagenase treatment: immunolocalization of tubulin, microtubule-associated proteins 1 and 2, plectin, vimentin, and vinculin". European Journal of Cell Biology. 38 (1): 113–22. PMID 2992982.
- Hijikata T, Murakami T, Imamura M, Fujimaki N, Ishikawa H (Mar 1999). "Plectin is a linker of intermediate filaments to Z-discs in skeletal muscle fibers". Journal of Cell Science. 112 (6): 867–76. PMID 10036236.
- Steinböck FA, Wiche G (Feb 1999). "Plectin: a cytolinker by design". Biological Chemistry. 380 (2): 151–8. doi:10.1515/BC.1999.023. PMID 10195422.
- Herrmann H, Wiche G (Jan 1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". The Journal of Biological Chemistry. 262 (3): 1320–5. PMID 3027087.
- Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S (Jul 2001). "Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin". Journal of Immunology. 167 (2): 641–5. doi:10.4049/jimmunol.167.2.641. PMID 11441066.
- Favre B, Schneider Y, Lingasamy P, Bouameur JE, Begré N, Gontier Y, Steiner-Champliaud MF, Frias MA, Borradori L, Fontao L (May 2011). "Plectin interacts with the rod domain of type III intermediate filament proteins desmin and vimentin". European Journal of Cell Biology. 90 (5): 390–400. doi:10.1016/j.ejcb.2010.11.013. PMID 21296452.
- Andrä K, Lassmann H, Bittner R, Shorny S, Fässler R, Propst F, Wiche G (Dec 1997). "Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture". Genes & Development. 11 (23): 3143–56. doi:10.1101/gad.11.23.3143. PMC 316746. PMID 9389647.
- Reipert S, Steinböck F, Fischer I, Bittner RE, Zeöld A, Wiche G (Nov 1999). "Association of mitochondria with plectin and desmin intermediate filaments in striated muscle". Experimental Cell Research. 252 (2): 479–91. doi:10.1006/excr.1999.4626. PMID 10527638.
- Huber O (Sep 2003). "Structure and function of desmosomal proteins and their role in development and disease". Cellular and Molecular Life Sciences. 60 (9): 1872–90. doi:10.1007/s00018-003-3050-7. PMID 14523549.
- Sonnenberg A, Liem RK (Jun 2007). "Plakins in development and disease". Experimental Cell Research. 313 (10): 2189–203. doi:10.1016/j.yexcr.2007.03.039. PMID 17499243.
- Thorolfsdottir, Rosa B.; Sveinbjornsson, Gardar; Sulem, Patrick; Helgadottir, Anna; Gretarsdottir, Solveig; Benonisdottir, Stefania; Magnusdottir, Audur; Davidsson, Olafur B.; Rajamani, Sridharan; Roden, Dan M.; Darbar, Dawood; Pedersen, Terje R.; Sabatine, Marc S.; Jonsdottir, Ingileif; Arnar, David O.; Thorsteinsdottir, Unnur; Gudbjartsson, Daniel F.; Holm, Hilma; Stefansson, Kari (2017). "A Missense Variant in PLEC Increases Risk of Atrial Fibrillation". Journal of the American College of Cardiology. 70 (17): 2157–2168. doi:10.1016/j.jacc.2017.09.005. PMC 5704994. PMID 29050564.
- Villa CR, Ryan TD, Collins JJ, Taylor MD, Lucky AW, Jefferies JL (Feb 2015). "Left ventricular non-compaction cardiomyopathy associated with epidermolysis bullosa simplex with muscular dystrophy and PLEC1 mutation". Neuromuscular Disorders. 25 (2): 165–8. doi:10.1016/j.nmd.2014.09.011. PMID 25454730.
- Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, Depinho RA, Mahmood U, Weissleder R (Apr 2008). "Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma". PLOS Medicine. 5 (4): e85. doi:10.1371/journal.pmed.0050085. PMC 2292750. PMID 18416599.
- Bausch D, Thomas S, Mino-Kenudson M, Fernández-del CC, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA (Jan 2011). "Plectin-1 as a novel biomarker for pancreatic cancer". Clinical Cancer Research. 17 (2): 302–9. doi:10.1158/1078-0432.CCR-10-0999. PMC 3044444. PMID 21098698.
Further reading
- Pfendner E, Rouan F, Uitto J (Apr 2005). "Progress in epidermolysis bullosa: the phenotypic spectrum of plectin mutations". Experimental Dermatology. 14 (4): 241–9. doi:10.1111/j.0906-6705.2005.00324.x. PMID 15810881.
- Foisner R, Traub P, Wiche G (May 1991). "Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin". Proceedings of the National Academy of Sciences of the United States of America. 88 (9): 3812–6. Bibcode:1991PNAS...88.3812F. doi:10.1073/pnas.88.9.3812. PMC 51543. PMID 2023931.
- Herrmann H, Wiche G (Jan 1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". The Journal of Biological Chemistry. 262 (3): 1320–5. PMID 3027087.
- Malecz N, Foisner R, Stadler C, Wiche G (Apr 1996). "Identification of plectin as a substrate of p34cdc2 kinase and mapping of a single phosphorylation site". The Journal of Biological Chemistry. 271 (14): 8203–8. doi:10.1074/jbc.271.14.8203. PMID 8626512.
- Liu CG, Maercker C, Castañon MJ, Hauptmann R, Wiche G (Apr 1996). "Human plectin: organization of the gene, sequence analysis, and chromosome localization (8q24)". Proceedings of the National Academy of Sciences of the United States of America. 93 (9): 4278–83. Bibcode:1996PNAS...93.4278L. doi:10.1073/pnas.93.9.4278. PMC 39526. PMID 8633055.
- Gache Y, Chavanas S, Lacour JP, Wiche G, Owaribe K, Meneguzzi G, Ortonne JP (May 1996). "Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy". The Journal of Clinical Investigation. 97 (10): 2289–98. doi:10.1172/JCI118671. PMC 507309. PMID 8636409.
- Smith FJ, Eady RA, Leigh IM, McMillan JR, Rugg EL, Kelsell DP, Bryant SP, Spurr NK, Geddes JF, Kirtschig G, Milana G, de Bono AG, Owaribe K, Wiche G, Pulkkinen L, Uitto J, McLean WH, Lane EB (Aug 1996). "Plectin deficiency results in muscular dystrophy with epidermolysis bullosa". Nature Genetics. 13 (4): 450–7. doi:10.1038/ng0896-450. PMID 8696340.
- McLean WH, Pulkkinen L, Smith FJ, Rugg EL, Lane EB, Bullrich F, Burgeson RE, Amano S, Hudson DL, Owaribe K, McGrath JA, McMillan JR, Eady RA, Leigh IM, Christiano AM, Uitto J (Jul 1996). "Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization". Genes & Development. 10 (14): 1724–35. doi:10.1101/gad.10.14.1724. PMID 8698233.
- Nikolic B, Mac Nulty E, Mir B, Wiche G (Sep 1996). "Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions". The Journal of Cell Biology. 134 (6): 1455–67. doi:10.1083/jcb.134.6.1455. PMC 2121005. PMID 8830774.
- Pulkkinen L, Smith FJ, Shimizu H, Murata S, Yaoita H, Hachisuka H, Nishikawa T, McLean WH, Uitto J (Oct 1996). "Homozygous deletion mutations in the plectin gene (PLEC1) in patients with epidermolysis bullosa simplex associated with late-onset muscular dystrophy". Human Molecular Genetics. 5 (10): 1539–46. doi:10.1093/hmg/5.10.1539. PMID 8894687.
- Gress TM, Müller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, Büchler M, Adler G, Lehrach H (Oct 1996). "A pancreatic cancer-specific expression profile". Oncogene. 13 (8): 1819–30. PMID 8895530.
- Andrä K, Nikolic B, Stöcher M, Drenckhahn D, Wiche G (Nov 1998). "Not just scaffolding: plectin regulates actin dynamics in cultured cells". Genes & Development. 12 (21): 3442–51. doi:10.1101/gad.12.21.3442. PMC 317224. PMID 9808630.
- Banwell BL, Russel J, Fukudome T, Shen XM, Stilling G, Engel AG (Aug 1999). "Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency". Journal of Neuropathology and Experimental Neurology. 58 (8): 832–46. doi:10.1097/00005072-199908000-00006. PMID 10446808.
- Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A (Oct 1999). "Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding". The Journal of Cell Biology. 147 (2): 417–34. doi:10.1083/jcb.147.2.417. PMC 2174221. PMID 10525545.
- Stegh AH, Herrmann H, Lampel S, Weisenberger D, Andrä K, Seper M, Wiche G, Krammer PH, Peter ME (Aug 2000). "Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis". Molecular and Cellular Biology. 20 (15): 5665–79. doi:10.1128/MCB.20.15.5665-5679.2000. PMC 86037. PMID 10891503.
- Bauer JW, Rouan F, Kofler B, Rezniczek GA, Kornacker I, Muss W, Hametner R, Klausegger A, Huber A, Pohla-Gubo G, Wiche G, Uitto J, Hintner H (Feb 2001). "A compound heterozygous one amino-acid insertion/nonsense mutation in the plectin gene causes epidermolysis bullosa simplex with plectin deficiency". The American Journal of Pathology. 158 (2): 617–25. doi:10.1016/S0002-9440(10)64003-5. PMC 1850321. PMID 11159198.
- Henzler T, Harmache A, Herrmann H, Spring H, Suzan M, Audoly G, Panek T, Bosch V (Mar 2001). "Fully functional, naturally occurring and C-terminally truncated variant human immunodeficiency virus (HIV) Vif does not bind to HIV Gag but influences intermediate filament structure". The Journal of General Virology. 82 (Pt 3): 561–73. doi:10.1099/0022-1317-82-3-561. PMID 11172097.
- Nakano A, Pulkkinen L, Murrell D, Rico J, Lucky AW, Garzon M, Stevens CA, Robertson S, Pfendner E, Uitto J (May 2001). "Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the beta 4 integrin gene (ITGB4) and genotype/phenotype correlations". Pediatric Research. 49 (5): 618–26. doi:10.1203/00006450-200105000-00003. PMID 11328943.
- Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S (Jul 2001). "Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin". Journal of Immunology. 167 (2): 641–5. doi:10.4049/jimmunol.167.2.641. PMID 11441066.
- Koss-Harnes D, Høyheim B, Anton-Lamprecht I, Gjesti A, Jørgensen RS, Jahnsen FL, Olaisen B, Wiche G, Gedde-Dahl T (Jan 2002). "A site-specific plectin mutation causes dominant epidermolysis bullosa simplex Ogna: two identical de novo mutations". The Journal of Investigative Dermatology. 118 (1): 87–93. doi:10.1046/j.0022-202x.2001.01591.x. PMID 11851880.
External links
- Mass spectrometry characterization of one isoform of PLEC at COPaKB.[1]
- GeneReviews/NCBI/NIH/UW entry on Epidermolysis Bullosa with Pyloric Atresia
- plectin at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Plectin". The University of Edinburgh. 2003-11-13. Retrieved 2008-02-17.
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (Oct 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–1053. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.