Cinnamaldehyde
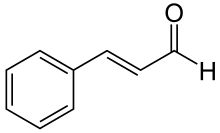
Cinnamaldehyde is an organic compound with the formula C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor.[1] It is a phenylpropanoid that is naturally synthesized by the shikimate pathway.[2] This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde.[3]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-Phenylprop-2-enal | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 1071571 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.111.079 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O | |
| Molar mass | 132.16 g/mol |
| Appearance | Yellow oil |
| Odor | Pungent, cinnamon-like |
| Density | 1.0497 g/mL |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 248 °C (478 °F; 521 K) |
| Slightly soluble | |
| Solubility |
|
| −7.48×10−5 cm3/mol | |
Refractive index (nD) |
1.6195 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H317, H319, H335 |
| P261, P264, P271, P272, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 71 °C (160 °F; 344 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3400 mg/kg (rat, oral) |
| Related compounds | |
Related compounds |
Cinnamic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and synthesis
Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot[4] and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854.[5]
The natural product is trans-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. As such, the molecule can be viewed as a derivative of acrolein. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towards the visible.[6]
Biosynthesis

The biosynthesis of cinnamaldehyde begins with deamination of L-phenylalanine into cinnamic acid by the action of phenylalanine ammonia lyase (PAL).[7][8] PAL catalyzes this reaction by a non-oxidative deamination. This deamination relies on the MIO prosthetic group of PAL.[9] PAL gives rise to trans-cinnamic acid.
In the second step, 4-coumarate–CoA ligase (4CL) converts cinnamic acid to cinnamoyl-CoA by an acid–thiol ligation.[7] 4CL uses ATP to catalyze the formation of cinnamoyl-CoA.[10] 4CL effects this reaction in two steps.[11] 4CL forms a hydroxycinnamate–AMP anhydride, followed by a nucleophile attack on the carbonyl of the acyl adenylate.[12]
Cinnamoyl-CoA is reduced by NADPH catalyzed by CCR (cinnamoyl-CoA reductase) to form cinnamaldehyde.[7][13]
Synthesis
Several methods of laboratory synthesis exist, but cinnamaldehyde is most economically obtained from the steam distillation of the oil of cinnamon bark. The compound can be prepared from related compounds such as cinnamyl alcohol, (the alcohol form of cinnamaldehyde), but the first synthesis from unrelated compounds was the aldol condensation of benzaldehyde and acetaldehyde.
Metabolism
Cinnamaldehyde occurs widely, and closely related compounds give rise to lignin. All such compounds are biosynthesized starting from phenylalanine, which undergoes conversion.[14]
Cinnamoyl-CoA reductase is an enzyme responsible for the production of cinnamoyl-CoA from cinnamaldehyde.
Autoxidation produces cinnamic acid.
Applications
As a flavorant
The most obvious application for cinnamaldehyde is as flavoring in chewing gum, ice cream, candy, eliquid and beverages; use levels range from 9 to 4,900 parts per million (ppm) (that is, less than 0.5%). It is also used in some perfumes of natural, sweet, or fruity scents. Almond, apricot, butterscotch, and other aromas may partially employ the compound for their pleasant smells. Cinnamaldehyde can be used as a food adulterant; powdered beechnut husk aromatized with cinnamaldehyde can be marketed as powdered cinnamon.[15] Some breakfast cereals contain as much as 187 ppm cinnamaldehyde.[16]
As an agrichemical
Cinnamaldehyde has been tested as a safe and effective insecticide against mosquito larvae.[17] A concentration of 29 ppm of cinnamaldehyde kills half of Aedes aegypti mosquito larvae in 24 hours.[18] Trans-cinnamaldehyde works as a potent fumigant and practical repellant for adult mosquitos.[19]
Miscellaneous uses
Cinnamaldehyde is also known as a corrosion inhibitor for steel and other ferrous alloys in corrosive fluids such as hydrochloric acid. It is believed that this is achieved by polymerization to form a protective film on the metal surface.[20][21] It can be used in combination with additional components such as dispersing agents, solvents and other surfactants. Cinnamaldehyde is also a potent inducer of apoptosis via ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells.[22] Some early evidence shows that cinnamaldehyde blocks formation of Tau protein aggregation into neurofibrillary tangles, a major pathology in Alzheimer's Disease. [23] Cinnamaldehyde also has antimicrobial properties.[24] Cinnamaldehyde is also a TRPA1 activator, and can excite a subset of sensory neurons that are mainly cold-sensitive neurons, to cause nociceptive behavior in mice.[25] Cinnamaldehyde has been found to improve metabolic health by acting directly on adipocytes and inducing them to start burning energy through a process called thermogenesis.[26][27] Scientists had previously observed that cinnamaldehyde appeared to protect mice against obesity and hyperglycemia, but the mechanisms underlying these effects were not well understood. Researchers are currently investigating cinnamaldehyde as a potential anti-obesity drug.
Derivatives
Numerous derivatives of cinnamaldehyde are commercially useful. Dihydrocinnamyl alcohol, which occurs naturally but is produced by double hydrogenation of cinnamaldehyde, is used to confer the fragrances of hyacinth and lilac. Cinnamyl alcohol similarly occurs naturally and has the odor of lilac, but can be also produced starting from cinnamaldehyde.[28] Dihydrocinnamaldehyde is produced by the selective hydrogenation of the alkene subunit. α-Amylcinnamaldehyde and α-hexylcinnamaldehyde are important commercial fragrances, but they are not prepared from cinnamaldehyde.[15]
Toxicology
Cinnamaldehyde is used in agriculture because of its low toxicity, but it is a skin irritant.[29]
References
- "Cinnamon". Transport Information Service. Gesamtverband der Deutschen Versicherungswirtschaft e.V. Retrieved 2007-10-23.
- Gutzeit, Herwig (2014). Plant Natural Products: Synthesis, Biological Functions and Practical Applications. Wiley. pp. 19–21. ISBN 978-3-527-33230-4.
- PubChem. "Cinnamaldehyde". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-10-18.
- Dumas, J.; Péligot, E. (1834). "Recherches de Chimie organique. — Sur l'Huile de Cannelle, l'Acide hippurique et l'Acide sébacique" [Organic chemistry research – On cinnamon oil, hippuric acid and sebacic acid]. Annales de Chimie et de Physique (in French). 57: 305–334.
- Chiozza, L. (1856). "Sur la production artificielle de l'essence de cannelle" [On the artificial production of cinnamon oil]. Comptes Rendus (in French). 42: 222–227.
- Inuzuka, Kozo (1961). "π Electronic structure of cinnamaldehyde". Bulletin of the Chemical Society of Japan. 34 (11): 1557–60. doi:10.1246/bcsj.34.1557.
- Bang, Hyun-bae; Lee, Yoon-hyeok; Kim, Sun-chang; Sung, Chang-keun; Jeong, Ki-jun (2016-01-19). "Metabolic engineering of Escherichia coli for the production of cinnamaldehyde". Microbial Cell Factories. 15 (1): 16. doi:10.1186/s12934-016-0415-9. ISSN 1475-2859. PMC 4719340. PMID 26785776.
- Koukol, J.; Conn, E. E. (1961-10-01). "The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare". The Journal of Biological Chemistry. 236: 2692–2698. ISSN 0021-9258. PMID 14458851.
- Kong, Jian-Qiang (2015-07-20). "Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering". RSC Advances. 5 (77): 62587–62603. doi:10.1039/C5RA08196C. ISSN 2046-2069.
- Beuerle, Till; Pichersky, Eran (2002-03-15). "Enzymatic Synthesis and Purification of Aromatic Coenzyme A Esters". Analytical Biochemistry. 302 (2): 305–312. doi:10.1006/abio.2001.5574. PMID 11878812.
- Allina, Sandra M.; Pri-Hadash, Aviva; Theilmann, David A.; Ellis, Brian E.; Douglas, Carl J. (1998-02-01). "4-Coumarate:Coenzyme A Ligase in Hybrid Poplar". Plant Physiology. 116 (2): 743–754. doi:10.1104/pp.116.2.743. ISSN 0032-0889. PMC 35134. PMID 9489021.
- Li, Zhi; Nair, Satish K. (2015-11-03). "Structural Basis for Specificity and Flexibility in a Plant 4-Coumarate:CoA Ligase". Structure. 23 (11): 2032–2042. doi:10.1016/j.str.2015.08.012. ISSN 1878-4186. PMID 26412334.
- Wengenmayer, Herta; Ebel, Jurgen; Grisebach, Hans (1976). "Enzymic Synthesis of Lignin Precursors. Purification and Properties of a Cinnamoyl-CoA:NADPH Reductase from Cell Suspension Cultures of Soybean (Glycine max)". European Journal of Biochemistry. 65 (2): 529–536. doi:10.1111/j.1432-1033.1976.tb10370.x. ISSN 0014-2956. PMID 7454.
- Boerjan, Wout; Ralph, John; Baucher, Marie (2003). "Ligninbiosynthesis". Annual Review of Plant Biology. 54: 519–546. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.
- Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- Friedman, M.; Kozuekue, N.; Harden, L. A. (2000). "Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry". Journal of Agricultural and Food Chemistry. 48 (11): 5702–5709. doi:10.1021/jf000585g. PMID 11087542.
- Dick-Pfaff, Cornelia (July 19, 2004). "Wohlriechender Mückentod" (in German).
- Cheng, Sen-Sung; Liu, Ju-Yun; Tsai, Kun-Hsien; Chen, Wei-June; Chang, Shang-Tzen (2004). "Chemical Composition and Mosquito Larvicidal Activity of Essential Oils from Leaves of Different Cinnamomum osmophloeum Provenances". Journal of Agricultural and Food Chemistry. 52 (14): 4395–4400. doi:10.1021/jf0497152. PMID 15237942. Lay summary – Science Daily (July 16, 2004).
- Ma, W.-B.; Feng, J.-T.; Jiang, Z.-L.; Zhang, X. (2014). "Fumigant Activity of 6 Selected Essential Oil Compounds and Combined Effect of Methyl Salicylate And trans-Cinnamaldehyde Against Culex pipiens pallens". Journal of the American Mosquito Control Association. 30 (3): 199–203. doi:10.2987/14-6412R.1. PMID 25843095.
- Growcock, F. B. (1989). "Inhibition of Steel Corrosion in HCl by Derivatives of Cinnamaldehyde". Corrosion. 45 (12): 1003–1007. doi:10.5006/1.3585007.
- Growcock, F. B.; Frenier, W. W.; Andreozzi, P. A. (1989). "Inhibition of Steel Corrosion in HCl by Derivatives of Cinnamaldehyde". Corrosion. 45 (12): 1007–1015. doi:10.5006/1.3585008.
- Ka, Hyeon; Park, Hee-Juhn; Jung, Hyun-Ju; Choi, Jong-Won; Cho, Kyu-Seok; Ha, Joohun; Lee, Kyung-Tae (2003). "Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells". Cancer Letters. 196 (2): 143–152. doi:10.1016/s0304-3835(03)00238-6. PMID 12860272.
- George, R. C. (2013). "Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer's disease pathogenesis". Journal of Alzheimer's Disease. 36 (3/1/2013): 21–40. doi:10.3233/JAD-122113. PMID 23531502. Retrieved 22 May 2018.
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. (2007). "Vapor-Phase Activities of Cinnamon, Thyme, and Oregano Essential Oils and Key Constituents against Foodborne Microorganisms". J. Agric. Food Chem. 55 (11): 4348–4356. doi:10.1021/jf063295u. PMID 17488023.
- Bandell, M; Story, G. M.; Hwang, S. W.; Viswanath, V.; Eid, S. R.; Petrus, M. J.; Earley, T. J.; Patapoutian, A. (2004). "Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin". Neuron. 41 (6): 849–857. doi:10.1016/s0896-6273(04)00150-3. PMID 15046718.
- Jiang, Juan; Emont, Margo P.; Jun, Heejin; Qiao, Xiaona; Liao, Jiling; Kim, Dong-il; Wu, Jun (December 2017). "Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming". Metabolism. 77: 58–64. doi:10.1016/j.metabol.2017.08.006. PMC 5685898. PMID 29046261.
- University of Michigan (November 21, 2017). "Cinnamon turns up the heat on fat cells".
- Zucca, P.; Littarru, M.; Rescigno, A.; Sanjust, E. (2009). "Cofactor recycling for selective enzymatic biotransformation of cinnamaldehyde to cinnamyl alcohol". Bioscience, Biotechnology, and Biochemistry. 73 (5): 1224–1226. doi:10.1271/bbb.90025. PMID 19420690.
- Olsen, R. V.; Andersen, H. H.; Møller, H. G.; Eskelund, P. W.; Arendt-Nielsen, L (2014). "Somatosensory and vasomotor manifestations of individual and combined stimulation of TRPM8 and TRPA1 using topical L-menthol and trans-cinnamaldehyde in healthy volunteers". European Journal of Pain. 18 (9): 1333–42. doi:10.1002/j.1532-2149.2014.494.x. PMID 24664788.
