Thiazide
Thiazide (/ˈθaɪəzaɪd/) refers to both a class of sulfur-containing organic molecules[1] and a class of diuretics based on the chemical structure of benzothiadiazine.[2] The thiazide drug class was discovered and developed at Merck and Co. in the 1950s.[3] The first approved drug of this class, chlorothiazide, was marketed under the trade name Diuril beginning in 1958.[3] In most countries, thiazides are the least expensive antihypertensive drugs available.[4]
(1%2C2%2C4)thiadiazine_200.svg.png)

Thiazide organic molecules are bi-cyclic structures that contain adjacent sulfur and nitrogen atoms on one ring.[5] Confusion sometimes occurs because thiazide-like diuretics such as indapamide are referred to as thiazides despite not having the thiazide chemical structure.[6] When used this way, "thiazide" refers to a drug which acts at the thiazide receptor.[7] The thiazide receptor is a sodium-chloride transporter that pulls NaCl from the lumen in the distal convoluted tubule. Thiazide diuretics inhibit this receptor, causing the body to release NaCl and water into the lumen, thereby increasing the amount of urine produced each day.[6] An example of a molecule that is chemically a thiazide but not used as a diuretic is methylchloroisothiazolinone, often found as an antimicrobial in cosmetics.[8]
Medical uses
Thiazide diuretics are primary used to treat the hypertension (high blood pressure) and edema (swelling) caused by water overload as well as certain conditions related to unbalanced calcium metabolism.
Water balance
Hypertension
There are many causes of hypertension (high blood pressure), including advancing age, smoking and obesity.[9] Sometimes the underlying cause of hypertension can not be determined, resulting in a diagnosis of idiopathic hypertension. Regardless of the cause, someone may have very high hypertension without any initial symptoms. Uncontrolled hypertension will eventually cause damage the heart, kidneys and eyes. Lifestyle changes, including reducing dietary salt, increasing exercise and losing weight can help to reduce blood pressure.[9]
Thiazides and thiazide-like diuretics have been in constant use since their introduction in 1958. Decades as a cornerstone of hypertension treatment show how well these drugs perform for most patients.[10] Low-dose thiazides are tolerated as well as the other classes of diuretics, including ACE inhibitors, beta blockers and calcium channel blockers.[9] In general, the thiazides and thiazide-like diuretics reduce the risk of death, stroke, heart attack, and heart failure due to hypertension.[11]
Clinical practice guidelines regarding the use of thiazides vary by geographic region. Guidelines in the United States recommend thiazides as a first-line treatment for hypertension (JNC VIII).[12] A systematic review by the Cochrane Collaboration specifically recommended that low-dose thiazides be used as the initial pharmacological therapy for high blood pressure.[9] Low-dose thiazides are more effective at treating hypertension than beta blockers and are similar to angiotensin-converting enzyme (ACE) inhibitors.[9] Thiazides are a recommended treatment for hypertension in Europe (ESC/ESH).[13] However, the UK National Institute for Health and Clinical Excellence recommends ACE inhibitor and calcium channel blockers for first-line treatment of hypertension in adults (CG127).[14] Thiazides should be considered as initial treatment if the patient has a high risk of developing heart failure.[14] Thiazides have also been replaced by ACE inhibitors in Australia due to the association between thaizide use and increased risk of developing diabetes mellitus type 2.[15]
| Drug Type | Generic Drug Name | Low Dose
Threshold (mg/day)[9] |
|---|---|---|
| Thiazide Diuretic | Chlorothiazide | 500 |
| Hydrochlorothiazide | 50 | |
| Bendroflumethiazide | 5 | |
| Methyclothiazide | 5 | |
| Trichlormethiazide | 2 | |
| Thiazide-like Diuretic | Chlortalidone | 50 |
| Indapamide | 5 |
Diabetes insipidus
Thiazides can be used to paradoxically decrease urine flow in people with nephrogenic diabetes insipidus.[16] Thiazides may also be useful in treating hyponatremia (low blood sodium) in infants with central diabetes insipidus.[17]
Calcium balance
Urinary stones
Thiazides are useful in treating kidney stones and bladder stones that result from hypercalciuria (high urine calcium levels). Thiazides increase the uptake of calcium in the distal tubules, to moderately reduce urinary calcium. Thiazides combined with potassium citrate, increased water intake and decreased dietary oxalate and sodium can slow or even reverse the formation of calcium-containing kidney stones.[18] High-dose therapy with the thiazide-like diuretic indapamide can be used to treat idiopathic hypercalcinuria (high urine calcium with unknown cause).[19]
Dent's disease
Thiazides may be used to treat the symptoms of Dent's disease, an X-linked genetic condition that results in electrolyte imbalance with repeated episodes of kidney stones. A case study of two brothers with the condition, two years of treatment with hydrochlorothiazide reduced the incidence of kidney stones and improved kidney function.[20] The thiazide-like diuretic chlortalidone reduced urine calcium oxalate in seven of the eight males with inactivated CLCN5 gene that participated in the study.[21] Inactivation of the CLCN5 gene causes Dent's disease Type 1.[22] The rare nature of Dent's disease makes it difficult to coordinate large controlled studies, so most evidence for thiazide use is with too few patients to make broad recommendations possible.[22] Long-term thiazide use may not be advisable due to the risk of significant adverse side effects.
Osteoporosis
Hypocalcemia (low blood calcium) can be caused by a variety of conditions that reduce dietary calcium absorption, increase calcium excretion or both. Positive calcium balance occurs when calcium excretion is decreased and intake remains constant so that calcium is retained within the body.[23] Higher levels of retained calcium are associated with increased bone mineral density and fewer fractures in individuals with osteoporosis.[23] By a poorly understood mechanism, thiazides directly stimulate osteoblast differentiation and bone mineral formation, further slowing the course of osteoporosis.[24]
Other uses
Bromine intoxication can be treated by giving intravenous saline with either thiazides or Loop diuretics.[25]
Contraindications
Contraindications include:
- Hypotension
- Allergy to sulphur-containing medications
- Gout
- Kidney failure
- Lithium therapy
- Hypokalemia
- May worsen diabetes
Thiazides reduce the clearance of uric acid since they compete for the same transporter, and therefore raise the levels of uric acid in the blood. Hence, they are prescribed with caution in patients with gout or hyperuricemia.[26][27]
Chronic administration of thiazides is associated with the increase of insulin resistance which can lead to hyperglycemia.[28]
Thiazides cause loss of blood potassium, while conserving blood calcium.
Thiazides can decrease placental perfusion and adversely affect the fetus, so should be avoided in pregnancy.[27][29]
Adverse effects
- Hypokalemia – Thiazide diuretics reduces potassium concentration in blood through two indirect mechanisms: inhibition of sodium-chloride symporter at distal convoluted tubule of a nephron and stimulation of aldosterone that activates Na+/K+-ATPase at collecting duct. Inhibition of sodium-chloride symporter increases availability of sodium and chloride in urine. When the urine reaches the collecting duct, the increase in sodium and chloride availability activates Na+/K+-ATPase, which increases the absorption of sodium and excretion of potassium into the urine. Long term administration of thiazide diuretics reduces total body blood volume. This activates the renin–angiotensin system, stimulates the secretion of aldosterone, thus activating Na+/K+-ATPase, increasing excretion of potassium in urine.[30] Therefore, ACE inhibitor and thiazide combination is used to prevent hypokalemia.
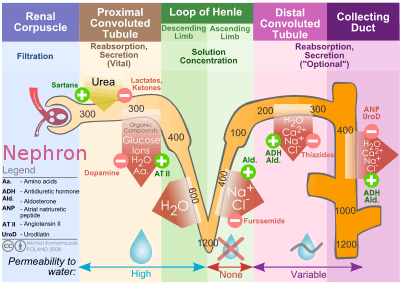 Overview of nephron function and where thiazide diuretics act.
Overview of nephron function and where thiazide diuretics act. - Hyperglycemia
- Hyperlipidemia
- Hyperuricemia
- Hypercalcemia
- Hyponatremia
- Hypomagnesemia
- Hypocalciuria
Mechanism of action
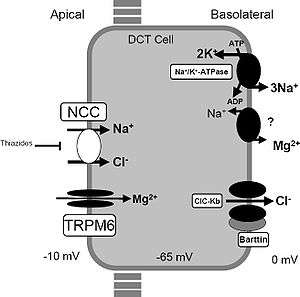
Thiazide diuretics control hypertension in part by inhibiting reabsorption of sodium (Na+) and chloride (Cl−) ions from the distal convoluted tubules in the kidneys by blocking the thiazide-sensitive Na+-Cl− symporter.[31] The term "thiazide" is also often used for drugs with a similar action that do not have the thiazide chemical structure, such as chlorthalidone and metolazone. These agents are more properly termed thiazide-like diuretics.
Thiazide diuretics also increase calcium reabsorption at the distal tubule. By lowering the sodium concentration in the tubule epithelial cells, thiazides indirectly increase the activity of the basolateral Na+/Ca2+ antiporter to maintain intracellular Na+ level, facilitating Ca2+ to leave the epithelial cells into the renal interstitium. Thus, intracellular Ca2+ concentration is decreased, which allows more Ca2+ from the lumen of the tubules to enter epithelial cells via apical Ca2+-selective channels (TRPV5). In other words, less Ca2+ in the cell increases the driving force for reabsorption from the lumen.[32]
Thiazides are also thought to increase the reabsorption of Ca2+ by a mechanism involving the reabsorption of sodium and calcium in the proximal tubule in response to sodium depletion. Some of this response is due to augmentation of the action of parathyroid hormone.[33]
Breastfeeding
Thiazides pass into breast milk and can decrease the flow of breast milk.[34] Thiazides have been associated with significant side effects in some nursing infants and should be administered to nursing mothers with caution.[35]
History
The thiazide diuretics were developed by scientists Karl H. Beyer, James M. Sprague, John E. Baer, and Frederick C. Novello of Merck and Co. in the 1950s,[36] and led to the marketing of the first drug of this class, chlorothiazide, under the trade name Diuril in 1958.[37] The research leading to the discovery of chlorothiazide, leading to "the saving of untold thousands of lives and the alleviation of the suffering of millions of victims of hypertension" was recognized by a special Public Health Award from the Lasker Foundation in 1975.[38]
References
- Thiazides at the US National Library of Medicine Medical Subject Headings (MeSH)
- Thiazide+Diuretics at the US National Library of Medicine Medical Subject Headings (MeSH)
- Beyer KH (September 1993). "Chlorothiazide. How the thiazides evolved as antihypertensive therapy". Hypertension. 22 (3): 388–91. doi:10.1161/01.hyp.22.3.388. PMID 8349332.
- Whitworth JA (November 2003). "2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension" (PDF). Journal of Hypertension. 21 (11): 1983–92. doi:10.1097/00004872-200311000-00002. PMID 14597836.
- "MeSH Browser". meshb.nlm.nih.gov. Retrieved 2019-07-22.
- Akbari, Pegah; Khorasani-Zadeh, Arshia (2019), "Thiazide Diuretics", StatPearls, StatPearls Publishing, PMID 30422513, retrieved 2019-07-18
- thiazide+receptor at the US National Library of Medicine Medical Subject Headings (MeSH)
- "6 Final Report on the Safety Assessment of Methylisothiazolinone and Methylchloroisothiazolinone". Journal of the American College of Toxicology. 11 (1): 75–128. 1992-01-01. doi:10.3109/10915819209141993. ISSN 0730-0913.
- Musini, Vijaya M; Gill, Rupam; Wright, James M (2018-04-18). "First‐line drugs for hypertension". The Cochrane Database of Systematic Reviews. 2018 (4): CD001841. doi:10.1002/14651858.CD001841.pub3. ISSN 1469-493X. PMC 6513559. PMID 29667175.
- Moser M, Feig PU (November 2009). "Fifty years of thiazide diuretic therapy for hypertension". Archives of Internal Medicine. JAMA. 169 (20): 1851–6. doi:10.1001/archinternmed.2009.342. PMID 19901136.
- Wright JM, Musini VM, Gill R (April 2018). "First-line drugs for hypertension". The Cochrane Database of Systematic Reviews. 4: CD001841. doi:10.1002/14651858.CD001841.pub3. PMC 6513559. PMID 29667175.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. (February 2014). "2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)". JAMA. 311 (5): 507–20. doi:10.1001/jama.2013.284427. PMID 24352797.
- "escardio.org". Archived from the original on 2008-05-17. Retrieved 2007-08-30.
- National Institute for Health and Clinical Excellence (NICE) guideline on the management of primary hypertension in adults (CG127) accessed 5/3/2012 at "Archived copy". Archived from the original on 2012-01-31. Retrieved 2012-03-05.CS1 maint: archived copy as title (link)
- Guide to management of hypertension 2008. National Heart Foundation Australia. 2008. accessed online at "Archived copy" (PDF). Archived from the original (PDF) on 2013-05-15. Retrieved 2013-07-10.CS1 maint: archived copy as title (link)
- Magaldi, Antonio J. (2000-12-01). "New insights into the paradoxical effect of thiazides in diabetes insipidus therapy". Nephrology Dialysis Transplantation. 15 (12): 1903–1905. doi:10.1093/ndt/15.12.1903. ISSN 0931-0509. PMID 11096127.
- Welch, Thomas R. (2015-09-01). "Diuretics for diabetes insipidus". The Journal of Pediatrics. 167 (3): 503–505. doi:10.1016/j.jpeds.2015.07.029. ISSN 0022-3476.
- "THIAZIDE DIURETICS FOR STONE PREVENTION | Kidney Stone Evaluation And Treatment Program". kidneystones.uchicago.edu. Retrieved 2019-07-22.
- Martins, M. C.; Meyers, A. M.; Whalley, N. A.; Margolius, L. P.; Buys, M. E. (1996). "Indapamide (Natrilix): the agent of choice in the treatment of recurrent renal calculi associated with idiopathic hypercalciuria". British Journal of Urology. 78 (2): 176–180. doi:10.1046/j.1464-410X.1996.00633.x. ISSN 1464-410X. PMID 8813907.
- Velasco, Nestor; Jayawardene, Satishkumar A.; Burgess, Helen K. (2001-07-01). "Dent's disease: can we slow its progression?". Nephrology Dialysis Transplantation. 16 (7): 1512–1513. doi:10.1093/ndt/16.7.1512. ISSN 0931-0509. PMID 11427657.
- Scheinman, Steven J.; Asplin, John; Ploutz-Snyder, Robert J.; Why, Scott Van; Goodyer, Paul; Blowey, Douglas; D’Mello, Richard G.; Schurman, Scott; Raja, Khalid A. (2002-12-01). "Responsiveness of Hypercalciuria to Thiazide in Dent's Disease". Journal of the American Society of Nephrology. 13 (12): 2938–2944. doi:10.1097/01.ASN.0000036869.82685.F6. ISSN 1046-6673. PMID 12444212.
- "Dent Disease". NORD (National Organization for Rare Disorders). Retrieved 2019-07-22.
- Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No.: CD005185. DOI: 10.1002/14651858.CD005185.pub2.
- Dvorak MM, De Joussineau C, Carter DH, Pisitkun T, Knepper MA, Gamba G, Kemp PJ, Riccardi D (September 2007). "Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone". Journal of the American Society of Nephrology. 18 (9): 2509–16. doi:10.1681/ASN.2007030348. PMC 2216427. PMID 17656470.
- Trump, D. L.; Hochberg, M. C. (April 1976). "Bromide intoxication". The Johns Hopkins Medical Journal. 138 (4): 119–123. ISSN 0021-7263. PMID 131871.
- http://www.medscape.com/viewarticle/421426
- "Archived copy". Archived from the original on 2010-10-09. Retrieved 2010-05-14.CS1 maint: archived copy as title (link)
- Rehman, Abdur; Setter, Stephen M.; Vue, Mays H. (2011-11-01). "Drug-Induced Glucose Alterations Part 2: Drug-Induced Hyperglycemia". Diabetes Spectrum. 24 (4): 234–238. doi:10.2337/diaspect.24.4.234. ISSN 1040-9165.
- http://www.merck.com/mmpe/sec18/ch261/ch261k.html
- Dowd, Frank J; Johnson, Bart; Mariotti, Angelo (3 September 2016). Pharmacology and Therapeutics for Dentistry - E-Book. Elsevier Health Sciences. pp. 324–326. ISBN 9780323445955. Retrieved 4 November 2017.
- Duarte JD, Cooper-DeHoff RM (June 2010). "Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics". Expert Rev Cardiovasc Ther. 8 (6): 793–802. doi:10.1586/erc.10.27. PMC 2904515. PMID 20528637.
- Longo, Dan L; et al. (2012). Harrison's Principals of Internal Medicine, Vol. 2. New York: McGraw-Hill. p. 2285. ISBN 978-0-07-174887-2.
- Longo, Dan L; et al. (2012). Harrison's Principals of Internal Medicine, Vol. 2. New York: McGraw-Hill. p. 3109. ISBN 978-0-07-174887-2.
- Gerald G. Briggs; Roger K. Freeman; Sumner J. Yaffe (2011). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. pp. 257–. ISBN 978-1-60831-708-0.
- American Academy of Pediatrics Committee on Drugs (September 2001). "Transfer of drugs and other chemicals into human milk". Pediatrics. 108 (3): 776–89. doi:10.1542/peds.108.3.776. PMID 11533352.
- Beyer KH (1993). "Chlorothiazide. How the thiazides evolved as antihypertensive therapy". Hypertension. 22 (3): 388–91. doi:10.1161/01.hyp.22.3.388. PMID 8349332.
- "Drugs@FDA: FDA Approved Drug Products".
- "The Lasker Foundation - Awards".