Schizophrenia
Schizophrenia is a mental illness characterized by continuous or relapsing episodes of psychosis.[5] Major symptoms include hallucinations (often hearing voices), delusions, and disorganized thinking.[7] Other symptoms include social withdrawal, decreased emotional expression, and apathy.[5][13] Symptoms typically come on gradually, begin in young adulthood, and in many cases never resolve.[3][7] There is no objective diagnostic test; diagnosis is based on observed behavior, a history that includes the person's reported experiences, and reports of others familiar with the person.[7] To be diagnosed with schizophrenia, symptoms and functional impairment need to be present for six months, (DSM-5), or one month, (ICD-11).[7][11] Many people with schizophrenia have other mental disorders that often includes an anxiety disorder such as panic disorder, an obsessive–compulsive disorder, or a substance use disorder.[7]
| Schizophrenia | |
|---|---|
 | |
| Cloth embroidered by a person diagnosed with schizophrenia | |
| Pronunciation |
|
| Specialty | Psychiatry |
| Symptoms | Hallucinations (usually hearing voices), delusions, confused thinking[2][3] |
| Complications | Suicide, heart disease, lifestyle diseases[4] |
| Usual onset | Ages 16 to 30[3] |
| Duration | Chronic[3] |
| Causes | Environmental and genetic factors[5] |
| Risk factors | Family history, cannabis use in adolescence, problems during pregnancy, childhood adversity, birth in late winter or early spring, older father, being born or raised in a city[5][6] |
| Diagnostic method | Based on observed behavior, reported experiences, and reports of others familiar with the person[7] |
| Differential diagnosis | Substance abuse, Huntington's disease, mood disorders (bipolar disorder), autism,[8] borderline personality disorder[9] |
| Management | Counseling, job training[2][5] |
| Medication | Antipsychotics[5] |
| Prognosis | 20 years shorter life expectancy[4][10] |
| Frequency | ~0.5%[11] |
| Deaths | ~17,000 (2015)[12] |
About 0.3% to 0.7% of people are affected by schizophrenia during their lifetime.[14] In 2017, there were an estimated 1.1 million new cases and in 2019 a total of 20 million cases globally.[15][2] Males are more often affected and on average have an earlier onset.[2] The causes of schizophrenia include genetic and environmental factors.[5] Genetic factors include a variety of common and rare genetic variants.[16] Possible environmental factors include being raised in a city, cannabis use during adolescence, infections, the ages of a person's mother or father, and poor nutrition during pregnancy.[5][17]
About half of those diagnosed with schizophrenia will have a significant improvement over the long term with no further relapses, and a small proportion of these will recover completely.[7][18] The other half will have a lifelong impairment,[19] and severe cases may be repeatedly admitted to hospital.[18] Social problems such as long-term unemployment, poverty, homelessness, exploitation, and victimization are common consequences of schizophrenia.[20][21] Compared to the general population, people with schizophrenia have a higher suicide rate (about 5% overall) and more physical health problems,[22][23] leading to an average decreased life expectancy of 20 years.[10] In 2015, an estimated 17,000 deaths were caused by schizophrenia.[12]
The mainstay of treatment is antipsychotic medication, along with counselling, job training, and social rehabilitation.[5] Up to a third of people do not respond to initial antipsychotics, in which case the antipsychotic clozapine may be used.[24] In situations where there is a risk of harm to self or others, a short involuntary hospitalization may be necessary.[25] Long-term hospitalization may be needed for a small number of people with severe schizophrenia.[26] In countries where supportive services are limited or unavailable, long-term hospital stays are more typical.[27]
Signs and symptoms
Schizophrenia is a mental disorder characterized by significant alterations in perception, thoughts, mood, and behavior.[28] Symptoms are described in terms of positive, negative, and cognitive symptoms.[3][29] The positive symptoms of schizophrenia are the same for any psychosis and are sometimes referred to as psychotic symptoms. These may be present in any of the different psychoses, and are often transient making early diagnosis of schizophrenia problematic. Psychosis noted for the first time in a person who is later diagnosed with schizophrenia is referred to as a first-episode psychosis (FEP).[30][31]
Positive symptoms
Positive symptoms are those symptoms that are not normally experienced, but are present in people during a psychotic episode in schizophrenia. They include delusions, hallucinations, and disorganized thoughts and speech, typically regarded as manifestations of psychosis.[30] Hallucinations most commonly involve the sense of hearing as hearing voices but can sometimes involve any of the other senses of taste, sight, smell, and touch.[32] They are also typically related to the content of the delusional theme.[33] Delusions are bizarre or persecutory in nature. Distortions of self-experience such as feeling as if one's thoughts or feelings are not really one's own, to believing that thoughts are being inserted into one's mind, sometimes termed passivity phenomena, are also common.[34] Thought disorders can include thought blocking, and disorganized speech – speech that is not understandable is known as word salad.[3][35] Positive symptoms generally respond well to medication,[5] and become reduced over the course of the illness, perhaps related to the age-related decline in dopamine activity.[7]
Negative symptoms
Negative symptoms are deficits of normal emotional responses or of other thought processes.[36] The five recognised domains of negative symptoms are: emotional blunting – showing flat expressions or little emotion; alogia – a poverty of speech; anhedonia – an inability to feel pleasure; asociality – the lack of desire to form relationships, and avolition – a lack of motivation and apathy.[37] Other related symptoms are social withdrawal,[38] self-neglect particularly in hygiene, and self-care, and loss of judgment.[36] Negative symptoms appear to contribute more to poor quality of life, functional impairment, and to the burden on others than do positive symptoms.[19][39] People with greater negative symptoms often have a history of poor adjustment before the onset of illness.[38] Negative symptoms are less responsive to medication, and are the most difficult to treat.[40][41]
Cognitive symptoms
Cognitive deficits are the earliest and most constantly found symptoms in schizophrenia. They are often evident long before the onset of illness in the prodromal stage, and may be present in early adolescence, or childhood.[42][43] They are a core feature but not considered to be core symptoms, as are positive and negative symptoms.[44][45] However, their presence and degree of dysfunction is taken as a better indicator of functionality than the presentation of core symptoms.[42] Cognitive deficits become worse at first episode psychosis but then return to baseline, and remain fairly stable over the course of the illness.[46][47]
The deficits in cognition are seen to drive the negative psychosocial outcome in schizophrenia, and are claimed to equate to a possible reduction in IQ from the norm of 100 to 70–85.[48][49] Cognitive deficits may be of neurocognition (nonsocial) or of social cognition.[50] Neurocognition is the ability to receive and remember information, and includes verbal fluency, memory, reasoning, problem solving, speed of processing, and auditory and visual perception.[47] Verbal memory and attention are seen to be the most affected.[51][49] Verbal memory impairment is associated with a decreased level of semantic processing (relating meaning to words).[52] Another memory impairment is that of episodic memory.[53] An impairment in visual perception that is consistently found in schizophrenia is that of visual backward masking.[47] Visual processing impairments include an inability to perceive complex visual illusions.[54] Social cognition is concerned with the mental operations needed to interpret, and understand the self and others in the social world.[50][47] This is also an associated impairment, and facial emotion perception is often found to be difficult.[55][56] Facial perception is critical for ordinary social interaction.[57] Cognitive impairments do not usually respond to antipsychotics, and there are a number of interventions that are used to try to improve them.
Onset
Onset typically occurs between the late teens and early 30s, with the peak incidence occurring in males in the early to mid twenties, and in females in the late twenties.[3][7][11] Onset before the age of 17 is known as early-onset,[58] and before the age of 13, as can sometimes occur is known as childhood schizophrenia or very early-onset.[59][7] A later stage of onset can occur between the ages of 40 and 60, known as late-onset schizophrenia.[50] A later onset over the age of 60 which may be difficult to differentiate as schizophrenia, is known as very-late-onset schizophrenia-like psychosis.[50] Late onset has shown that a higher rate of females are affected; they have less severe symptoms, and need lower doses of antipsychotics.[50] The earlier favouring of onset in males is later seen to be balanced by a post-menopausal increase in the development in females. Estrogen produced pre-menopause, has a dampening effect on dopamine receptors but its protection can be overridden by a genetic overload.[60] There has been a dramatic increase in the numbers of older adults with schizophrenia.[61] An estimated 70% of those with schizophrenia have cognitive deficits, and these are most pronounced in early onset, and late-onset illness.[50][62]
Onset may happen suddenly, or may occur after the slow and gradual development of a number of signs and symptoms in a period known as the prodromal stage.[7] Up to 75% of those with schizophrenia go through a prodromal stage.[63] The negative and cognitive symptoms in the prodrome can precede FEP by many months, and up to five years.[64][46] The period from FEP and treatment is known as the duration of untreated psychosis (DUP) which is seen to be a factor in functional outcome. The prodromal stage is the high-risk stage for the development of psychosis.[47] Since the progression to first episode psychosis, is not inevitable an alternative term is often preferred of at risk mental state[47] Recognition and early intervention at the prodromal stage would minimize the disruption to educational and social development associated with schizophrenia, and has been the focus of many studies.[64][46] It is suggested that the use of anti-inflammatory compounds such as D-serine may prevent the transition to schizophrenia.[46] Cognitive symptoms are not secondary to positive symptoms, or to the side effects of antipsychotics.[47]
Cognitive impairments in the prodromal stage become worse after first episode psychosis (after which they return to baseline and then remain fairly stable), making early intervention to prevent such transition of prime importance.[46] Early treatment with cognitive behavioral therapies are the gold standard.[64] Neurological soft signs of clumsiness and loss of fine motor movement are often found in schizophrenia, and these resolve with effective treatment of FEP.[65][11]
Causes
Genetic, environmental, and vulnerability factors are involved in the development of schizophrenia.[66][5] The interactions of these risk factors are complex, as numerous and diverse insults from conception to adulthood can be involved.[66] A genetic predisposition on its own, without interacting environmental factors, will not give rise to the development of schizophrenia.[67][66] Schizophrenia is described as a neurodevelopmental disorder that lacks a precise boundary in its definition.[68][67]
Genetic
Estimates of the heritability of schizophrenia are between 70% and 80%, which implies that 70% to 80% of the individual differences in risk to schizophrenia is associated with genetics.[69][16] These estimates vary because of the difficulty in separating genetic and environmental influences, and their accuracy has been queried.[70][71] The greatest risk factor for developing schizophrenia is having a first-degree relative with the disease (risk is 6.5%); more than 40% of identical twins of those with schizophrenia are also affected.[72] If one parent is affected the risk is about 13% and if both are affected the risk is nearly 50%.[69] However, DSM-5 points out that most people with schizophrenia have no family history of psychosis.[7] Results of candidate gene studies of schizophrenia have generally failed to find consistent associations,[73] and the genetic loci identified by genome-wide association studies as associated with schizophrenia explain only a small fraction of the variation in the disease.[74]
Many genes are known to be involved in schizophrenia, each with small effect and unknown transmission and expression.[16][75] The summation of these effect sizes into a polygenic risk score can explain at least 7% of the variability in liability for schizophrenia.[76] Around 5% of cases of schizophrenia are understood to be at least partially attributable to rare copy-number variations (CNVs); these structural variations are associated with known genomic disorders involving deletions at 22q11.2 (DiGeorge syndrome), duplications at 16p11.2 16p11.2 duplication (most frequently found) and deletions at 15q11.2 (Burnside-Butler syndrome).[77] Some of these CNVs increase the risk of developing schizophrenia by as much as 20-fold, and are frequently comorbid with autism and intellectual disabilities.[77]
The genes CRHR1 and CRHBP have been shown to be associated with a severity of suicidal behavior. These genes code for stress response proteins needed in the control of the HPA axis, and their interaction can affect this axis. Response to stress can cause lasting changes in the function of the HPA axis possibly disrupting the negative feedback mechanism, homeostasis, and the regulation of emotion leading to altered behaviors.[67]
The question of how schizophrenia could be primarily genetically influenced, given that people with schizophrenia have lower fertility rates, is a paradox. It is expected that genetic variants that increase the risk of schizophrenia would be selected against due to their negative effects on reproductive fitness. A number of potential explanations have been proposed, including that alleles associated with schizophrenia risk confers a fitness advantage in unaffected individuals.[78][79] While some evidence has not supported this idea,[71] others propose that a large number of alleles each contributing a small amount can persist.[80]
Environment
Environmental factors, each associated with a slight risk of developing schizophrenia in later life include oxygen deprivation, infection, prenatal maternal stress, and malnutrition in the mother during fetal development.[22] A risk is also associated with maternal obesity, in increasing oxidative stress, and dysregulating the dopamine and serotonin pathways.[81] Both maternal stress and infection have been demonstrated to alter fetal neurodevelopment through an increase of pro-inflammatory cytokines.[82] There is a slighter risk associated with being born in the winter or spring possibly due to vitamin D deficiency[83] or a prenatal viral infection.[72] Other infections during pregnancy or around the time of birth that have been linked to an increased risk include infections by Toxoplasma gondii and Chlamydia.[84] The increased risk is about five to eight percent.[85] Viral infections of the brain during childhood are also linked to a risk of schizophrenia during adulthood.[86]
Adverse childhood experiences (ACEs), severe forms of which are classed as childhood trauma, range from being bullied or abused, to the death of a parent.[87] Many adverse childhood experiences can cause toxic stress and increase the risk of psychosis.[88][89][87] Schizophrenia was the last diagnosis to benefit from the link made between ACEs and adult mental health outcomes.[90]
Living in an urban environment during childhood or as an adult has consistently been found to increase the risk of schizophrenia by a factor of two,[22][91] even after taking into account drug use, ethnic group, and size of social group.[92] A possible link between the urban environment and pollution has been suggested to be the cause of the elevated risk of schizophrenia.[93]
Other risk factors of importance include social isolation, immigration related to social adversity and racial discrimination, family dysfunction, unemployment, and poor housing conditions.[72][94] Having a father older than 40 years, or parents younger than 20 years are also associated with schizophrenia.[5][95]
Substance use
About half of those with schizophrenia use recreational drugs, including cannabis, nicotine, and alcohol excessively.[96][97] Use of stimulants such as amphetamine and cocaine can lead to a temporary stimulant psychosis, which presents very similarly to schizophrenia. Rarely, alcohol use can also result in a similar alcohol-related psychosis.[72][98] Drugs may also be used as coping mechanisms by people who have schizophrenia, to deal with depression, anxiety, boredom, and loneliness.[96][99] The use of cannabis and tobacco are not associated with the development of cognitive deficits, and sometimes a reverse relationship is found where their use improves these symptoms.[45] However, substance abuse is associated with an increased risk of suicide, and a poor response to treatment.[100]
Cannabis-use may be a contributory factor in the development of schizophrenia, potentially increasing the risk of the disease in those who are already at risk.[17] The increased risk may require the presence of certain genes within an individual.[17] Its use is associated with doubling the rate.[101] The use of more potent strains of cannabis having a high level of its active ingredient tetrahydrocannabinol (THC), increases the risk further. One of these strains is well known as skunk.[102][103]
Mechanisms
The mechanisms of schizophrenia are unknown, and a number of models have been put forward to explain the link between altered brain function and schizophrenia.[22] One of the most common is the dopamine model, which attributes psychosis to the mind's faulty interpretation of the misfiring of dopaminergic neurons.[104] This has been directly related to the symptoms of delusions and hallucinations.[22][105][106][107] Abnormal dopamine signaling has been implicated in schizophrenia based on the usefulness of medications that affect the dopamine receptor and the observation that dopamine levels are increased during acute psychosis.[108][109] A decrease in D1 receptors in the dorsolateral prefrontal cortex may also be responsible for deficits in working memory.[110][111]
Another hypothesis is the glutamate model that links alterations between glutamatergic neurotransmission and neural oscillations that affect connections between the thalamus and the cortex.[112] Studies have shown that a reduced expression of a glutamate receptor – NMDA receptor, and glutamate blocking drugs such as phencyclidine and ketamine can mimic the symptoms and cognitive problems associated with schizophrenia.[113][114][112] Post-mortem studies consistently find that a subset of these neurons fail to express GAD67 (GAD1),[115] in addition to abnormalities in brain morphometry. The subsets of interneurons that are abnormal in schizophrenia are responsible for the synchronizing of neural ensembles needed during working memory tasks. These give the neural oscillations produced as gamma waves that have a frequency of between 30 and 80 hertz. Both working memory tasks and gamma waves are impaired in schizophrenia, which may reflect abnormal interneuron functionality.[115][116][117][118]
There are often impairments in cognition, social skills, and motor skills before the onset of schizophrenia, which suggests a neurodevelopmental model.[119] Such frameworks have hypothesized links between these biological abnormalities and symptoms.[120] Furthermore, problems before birth such as maternal infection, maternal malnutrition and complications during pregnancy all increase risk for schizophrenia.[5][121] Schizophrenia usually emerges 18-25, an age period that overlaps with certain stages of neurodevelopment that are implicated in schizophrenia.[122]
Deficits in executive functions, such as planning, inhibition, and working memory, are pervasive in schizophrenia. Although these functions are dissociable, their dysfunction in schizophrenia may reflect an underlying deficit in the ability to represent goal related information in working memory, and to utilize this to direct cognition and behavior.[123][124] These impairments have been linked to a number of neuroimaging and neuropathological abnormalities. For example, functional neuroimaging studies report evidence of reduced neural processing efficiency, whereby the dorsolateral prefrontal cortex is activated to a greater degree to achieve a certain level of performance relative to controls on working memory tasks. These abnormalities may be linked to the consistent post-mortem finding of reduced neuropil, evidenced by increased pyramidal cell density and reduced dendritic spine density. These cellular and functional abnormalities may also be reflected in structural neuroimaging studies that find reduced grey matter volume in association with deficits in working memory tasks.[125]
Positive symptoms have been linked to reduced cortical thickness in the superior temporal gyrus.[126] Severity of negative symptoms has been linked to reduced thickness in the left medial orbitofrontal cortex.[127] Anhedonia, traditionally defined as a reduced capacity to experience pleasure, is frequently reported in schizophrenia. However, a large body of evidence suggests that hedonic responses are intact in schizophrenia,[128] and that what is reported to be anhedonia is a reflection of dysfunction in other processes related to reward.[129] Overall, a failure of reward prediction is thought to lead to impairment in the generation of cognition and behavior required to obtain rewards, despite normal hedonic responses.[130]
It has been hypothesized that in some people, development of schizophrenia is related to intestinal tract dysfunction such as seen with non-celiac gluten sensitivity or abnormalities in the gut microbiota.[131] A subgroup of persons with schizophrenia present an immune response to gluten differently from that found in people with celiac, with elevated levels of certain serum biomarkers of gluten sensitivity such as anti-gliadin IgG or anti-gliadin IgA antibodies.[132]
Another theory links abnormal brain lateralization to the development of being left-handed which is significantly more common in those with schizophrenia.[133] This abnormal development of hemispheric asymmetry is noted in schizophrenia.[134] Studies have concluded that the link is a true and verifiable effect that may reflect a genetic link between lateralization and schizophrenia.[133][135]
Bayesian models of brain functioning have been utilized to link abnormalities in cellular functioning to symptoms.[136][137] Both hallucinations and delusions have been suggested to reflect improper encoding of prior expectations, thereby causing expectation to excessively influence sensory perception and the formation of beliefs. In approved models of circuits that mediate predictive coding, reduced NMDA receptor activation, could in theory result in the positive symptoms of delusions and hallucinations.[138][139][140]
Diagnosis
There is no objective test or biomarker to confirm diagnosis. Psychoses can occur in several conditions and are often transient making early diagnosis of schizophrenia difficult. Psychosis noted for the first time in a person that is later diagnosed with schizophrenia is referred to as a first-episode psychosis (FEP).
Criteria
Schizophrenia is diagnosed based on criteria in either the Diagnostic and Statistical Manual of Mental Disorders (DSM) published by the American Psychiatric Association or the International Statistical Classification of Diseases and Related Health Problems (ICD) published by the World Health Organization. These criteria use the self-reported experiences of the person and reported abnormalities in behavior, followed by a psychiatric assessment. The mental status examination is an important part of the assessment.[141] An established tool for assessing the severity of positive and negative symptoms is the Positive and Negative Syndrome Scale (PANSS).[142] This has been seen to have shortcomings relating to negative symptoms, and other scales – the Clinical Assessment Interview for Negative Symptoms (CAINS), and the Brief Negative Symptoms Scale (BNSS) have been introduced.[41] The DSM-5, published in 2013, gives a Scale to Assess the Severity of Symptom Dimensions outlining eight dimensions of symptoms.[44]
DSM-5 states that to be diagnosed with schizophrenia, two diagnostic criteria have to be met over the period of one month, with a significant impact on social or occupational functioning for at least six months. One of the symptoms needs to be either delusions, hallucinations, or disorganized speech. A second symptom could be one of the negative symptoms, or severely disorganized or catatonic behaviour.[7] A different diagnosis of schizophreniform disorder can be made before the six months needed for the diagnosis of schizophrenia.[7]
In Australia the guideline for diagnosis is for six months or more with symptoms severe enough to affect ordinary functioning.[143] In the UK diagnosis is based on having the symptoms for most of the time for one month, with symptoms that significantly affect the ability to work, study, or to carry on ordinary daily living, and with other similar conditions ruled out.[144]
The ICD criteria are typically used in European countries; the DSM criteria are used predominantly in the United States and Canada, and are prevailing in research studies. In practice, agreement between the two systems is high.[145] The current proposal for the ICD-11 criteria for schizophrenia recommends adding self-disorder as a symptom.[34]
A major unresolved difference between the two diagnostic systems is that of the requirement in DSM of an impaired functional outcome. WHO for ICD argues that not all people with schizophrenia have functional deficits and so these are not specific for the diagnosis.[44]
Changes made
Both manuals have adopted the chapter heading of Schizophrenia spectrum and other psychotic disorders; ICD modifying this as Schizophrenia spectrum and other primary psychotic disorders.[44] The definition of schizophrenia remains essentially the same as that specified by the 2000 text revised DSM-IV (DSM-IV-TR). However, with the publication of DSM-5, the APA removed all sub-classifications of schizophrenia.[44] ICD-11 has also removed subtypes. The removed subtype from both, of catatonic has been relisted in ICD-11 as a psychomotor disturbance that may be present in schizophrenia.[44]
Another major change was to remove the importance previously given to Schneider's first-rank symptoms.[146] DSM-5 still uses the listing of schizophreniform disorder but ICD-11 no longer includes it.[44] DSM-5 also recommends that a better distinction be made between a current condition of schizophrenia and its historical progress, to achieve a clearer overall characterization.[146]
A dimensional assessment has been included in DSM-5 covering eight dimensions of symptoms to be rated (using the Scale to Assess the Severity of Symptom Dimensions) – these include the five diagnostic criteria plus cognitive impairments, mania, and depression.[44] This can add relevant information for the individual in regard to treatment, prognosis, and functional outcome; it also enables the response to treatment to be more accurately described.[44][147]
Two of the negative symptoms – avolition and diminished emotional expression, have been given more prominence in both manuals.[44]
Comorbidities
Many people with schizophrenia have one or more other disorders that often includes an anxiety disorder such as panic disorder, an obsessive-compulsive disorder, or a substance use disorder. These are separate disorders that need separate treatments.[7] Sleep disorders are commonly found with schizophrenia, and are early signs of illness and also of relapse.[148] Sleep disorders are linked with positive symptoms that include disorganized thinking and can adversely affect neocortical plasticity and cognition.[148] The consolidation of memories is disrupted in sleep disorders.[149] They are associated with severity of illness, a poor prognosis, and poor quality of life.[150][151] Sleep onset and maintenance insomnia is a common symptom, regardless of whether treatment has been received or not.[150] There is also a clozapine-induced somnolence. A related condition is antipsychotic-induced restless legs syndrome. Genetic variations have been found associated with these conditions involving the circadian rhythm, dopamine and histamine metabolism, and signal transduction.[152]
Differential diagnosis
Psychotic symptoms lasting less than a month may be diagnosed as brief psychotic disorder, and various conditions may be classed as psychotic disorder not otherwise specified; schizoaffective disorder is diagnosed if symptoms of mood disorder are substantially present alongside psychotic symptoms. If the psychotic symptoms are the direct physiological result of a general medical condition or a substance, then the diagnosis is one of a psychosis secondary to that condition. Schizophrenia is not diagnosed if symptoms of pervasive developmental disorder are present unless prominent delusions or hallucinations are also present.[7]
Psychotic symptoms may be present in several other conditions, and mental disorders, including bipolar disorder,[8] borderline personality disorder,[9] substance intoxication, substance-induced psychosis, and a number of drug withdrawal syndromes. Non-bizarre delusions are also present in delusional disorder, and social withdrawal in social anxiety disorder, avoidant personality disorder and schizotypal personality disorder. Schizotypal personality disorder has symptoms that are similar but less severe than those of schizophrenia.[7] Schizophrenia occurs along with obsessive-compulsive disorder (OCD) considerably more often than could be explained by chance, although it can be difficult to distinguish obsessions that occur in OCD from the delusions of schizophrenia.[153]
A more general medical and neurological examination may be needed to rule out medical illnesses which may rarely produce psychotic schizophrenia-like symptoms, such as metabolic disturbance, systemic infection, syphilis, HIV-associated neurocognitive disorder, epilepsy, limbic encephalitis, and brain lesions. Stroke, multiple sclerosis, hyperthyroidism, hypothyroidism, and dementias such as Alzheimer's disease, Huntington's disease, frontotemporal dementia, and the Lewy body dementias may also be associated with schizophrenia-like psychotic symptoms.[154] It may be necessary to rule out a delirium, which can be distinguished by visual hallucinations, acute onset and fluctuating level of consciousness, and indicates an underlying medical illness. Investigations are not generally repeated for relapse unless there is a specific medical indication or possible adverse effects from antipsychotic medication. In children hallucinations must be separated from typical childhood fantasies.[7]
Prevention
Prevention of schizophrenia is difficult as there are no reliable markers for the later development of the disorder.[155] There is tentative though inconclusive evidence for the effectiveness of early intervention to prevent schizophrenia in the prodrome phase.[156] There is some evidence that early intervention in those with first-episode psychosis may improve short-term outcomes, but there is little benefit from these measures after five years.[22] Cognitive behavioral therapy may reduce the risk of psychosis in those at high risk after a year[157] and is recommended in this group, by the National Institute for Health and Care Excellence (NICE).[28] Another preventive measure is to avoid drugs that have been associated with development of the disorder, including cannabis, cocaine, and amphetamines.[72]
Antipsychotics are prescribed following a first-episode psychosis, and following remission a preventive maintenance use is continued to avoid relapse. However, it is recognised that some people do recover following a single episode and that long-term use of antipsychotics will not be needed but there is no way of identifying this group.[158]
Management
The primary treatment of schizophrenia is the use of antipsychotic medications, often in combination with psychosocial interventions and social supports.[22][159] Community support services including drop-in centers, visits by members of a community mental health team, supported employment,[160] and support groups are common. The time between the onset of psychotic symptoms to being given treatment – the duration of untreated psychosis (DUP) is associated with a poorer outcome in both the short term and the long term.[161]
Voluntary or involuntary admittance to hospital may be needed to treat a severe episode, however, hospital stays are as short as possible. In the UK large mental hospitals termed asylums began to be closed down in the 1950s with the advent of antipsychotics, and with an awareness of the negative impact of long-term hospital stays on recovery.[20] This process was known as deinstitutionalization, and community and supportive services were developed in order to support this change. Many other countries followed suit with the US starting in the 60s.[162] There will still remain a few people who do not improve enough to be discharged.[20][26] In those countries that lack the necessary supportive and social services long-term hospital stays are more usual.[27]
Medication
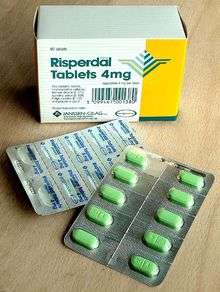
The first-line treatment for schizophrenia is an antipsychotic. The first-generation antipsychotics, now called typical antipsychotics, are dopamine antagonists that block D2 receptors, and affect the neurotransmission of dopamine. Those brought out later, the second-generation antipsychotics known as atypical antipsychotics, can also have effect on another neurotransmitter serotonin. Antipsychotics can reduce the symptoms of anxiety within hours of their use but for other symptoms they may take several days or weeks to reach their full effect.[30][163] They have little effect on negative and cognitive symptoms, which may be helped by additional psychotherapies and medications.[164] There is no single antipsychotic suitable for first-line treatment for everyone, as responses and tolerances vary between people.[165] Stopping medication may be considered after a single psychotic episode where there has been a full recovery with no symptoms for twelve months. Repeated relapses worsen the long-term outlook and the risk of relapse following a second episode is high, and long-term treatment is usually recommended.[166][167]
Tobacco smoking increases the metabolism of some antipsychotics, by strongly activitating CYP1A2 the enzyme that breaks them down, and a significant difference is found in these levels between smokers and non-smokers.[168][169][170] It is recommended that the dosage for those smokers on clozapine be increased by 50%, and for those on olanzapine by 30%.[169] The result of stopping smoking can lead to an increased concentration of the antipsychotic that may result in toxicity, so that monitoring of effects would need to take place with a view to decreasing the dosage; many symptoms may be noticeably worsened, and extreme fatigue, and seizures are also possible with a risk of relapse. Likewise those who resume smoking may need their dosages adjusted accordingly.[171][168] The altering effects are due to compounds in tobacco smoke and not to nicotine; the use of nicotine replacement therapy therefore has the equivalent effect of stopping smoking and monitoring would still be needed.[168]
About 30 to 50 percent of people with schizophrenia fail to accept that they have an illness or comply with their recommended treatment.[172] For those who are unwilling or unable to take medication regularly, long-acting injections of antipsychotics may be used,[173] which reduce the risk of relapse to a greater degree than oral medications.[174] When used in combination with psychosocial interventions, they may improve long-term adherence to treatment.[175]
Research findings suggested that other neurotransmission systems including serotonin, glutamate, GABA, and acetycholine were implicated in the development of schizophrenia, and that a more inclusive medication was needed.[170] A new first-in-class antipsychotic that targets multiple neurotransmitter systems called lumateperone (ITI-007), was trialed and approved by the FDA in December 2019 for the treatment of schizophrenia in adults.[176][177][170] Lumateperone is a small molecule agent that shows improved safety, and tolerance. It interacts with dopamine, serotonin, and glutamate in a complex, uniquely selective manner, and is seen to improve negative symptoms, and social functioning. Lumateperone was also found to reduce potential metabolic dysfunction, have lower rates of movement disorders, and have lower cardiovascular side effects such as a fast heart rate.[170]
Side effects
Typical antipsychotics are associated with a higher rate of movement disorders including akathisia. Some atypicals are associated with considerable weight gain, diabetes and the risk of metabolic syndrome.[178] Risperidone (atypical) has a similar rate of extrapyramidal symptoms to haloperidol (typical).[178] A rare but potentially lethal condition of neuroleptic malignant syndrome (NMS) has been associated with the use of antipsychotics. Through its early recognition, and timely intervention rates have declined. However, an awareness of the syndrome is advised to enable intervention.[179] Another less rare condition of tardive dyskinesia can occur due to long-term use of antipsychotics, developing after many months or years of use. It is more often reported with use of typical antipsychotics.[180]
Clozapine is associated with side effects that include weight gain, tiredness, and hypersalivation. More serious adverse effects include seizures, NMS, neutropenia, and agranulocytosis (lowered white blood cell count) and its use needs careful monitoring.[181][182] Studies have found that antipsychotic treatment following NMS and neutropenia may sometimes be successfully rechallenged (restarted) with clozapine.[183][184]
Clozapine is also associated with thromboembolism (including pulmonary embolism), myocarditis, and cardiomyopathy.[185][186] A systematic review of clozapine-associated pulmonary embolism indicates that this adverse effect can often be fatal, and that it has an early onset, and is dose-dependent. The findings advised the consideration of using a prevention therapy for venous thromboembolism after starting treatment with clozapine, and continuing this for six months.[186] Constipation is three times more likely to occur with the use of clozapine, and severe cases can lead to ileus and bowel ischemia resulting in many fatalities.[181]
However, the risk of serious adverse effects from clozapine is low, and there are the beneficial effects to be gained of a reduced risk of suicide, and aggression.[187][188] Typical antipsychotics and atypical risperidone can have a side effect of sexual dysfunction.[72] Clozapine, olanzapine, and quetiapine are associated with beneficial effects on sexual functioning helped by various psychotherapies.[189] Unwanted side effects cause people to stop treatment, resulting in relapses.[190]
Treatment resistant schizophrenia
About half of those with schizophrenia will respond favourably to antipsychotics, and have a good return of functioning.[191] However, positive symptoms persist in up to a third of people. Following two trials of different antipsychotics over six weeks, that also prove ineffective, they will be classed as having treatment resistant schizophrenia (TRS), and clozapine will be offered.[192][24] Clozapine is of benefit to around half of this group although it has the potentially serious side effect of agranulocytosis (lowered white blood cell count) in less than 4% of people.[22][72][193] Between 12 and 20 per cent will not respond to clozapine and this group is said to have ultra treatment resistant schizophrenia.[192][194] ECT may be offered to treat TRS as an add-on therapy, and is shown to sometimes be of benefit.[194] A review concluded that this use only has an effect on medium-term TRS and that there is not enough evidence to support its use other than for this group.[195]
TRS is often accompanied by a low quality of life, and greater social dysfunction.[196] TRS may be the result of inadequate rather than inefficient treatment; it also may be a false label due to medication not being taken regularly, or at all.[188] About 16 per cent of people who had initially been responsive to treatment later develop resistance. This could relate to the length of time on APs, with treatment becoming less responsive.[197] This finding also supports the involvement of dopamine in the development of schizophrenia.[188] Studies suggest that TRS may be a more heritable form.[198]
TRS may be evident from first episode psychosis, or from a relapse. It can vary in its intensity and response to other therapies.[196] This variation is seen to possibly indicate an underlying neurobiology such as dopamine supersensitivity (DSS), glutamate or serotonin dysfunction, inflammation and oxidative stress.[192] Studies have found that dopamine supersensitivity is found in up to 70% of those with TRS.[199] The variation has led to the suggestion that treatment responsive and treatment resistant schizophrenia be considered as two different subtypes.[192][198] It is further suggested that if the subtypes could be distinguished at an early stage significant implications could follow for treatment considerations, and for research.[194] Neuroimaging studies have found a significant decrease in the volume of grey matter in those with TRS with no such change seen in those who are treatment responsive.[194] In those with ultra treatment resistance the decrease in grey matter volume was larger.[192][194]
A link has been made between the gut microbiota and the development of TRS. The most prevalent cause put forward for TRS is that of mutation in the genes responsible for drug effectiveness. These include liver enzyme genes that control the availability of a drug to brain targets, and genes responsible for the structure and function of these targets. In the colon the bacteria encode a hundred times more genes than exist in the human genome. Only a fraction of ingested drugs reach the colon, having been already exposed to small intestinal bacteria, and absorbed in the portal circulation. This small fraction is then subject to the metabolic action of many communities of bacteria. Activation of the drug depends on the composition and enzymes of the bacteria and of the specifics of the drug, and therefore a great deal of individual variation can affect both the usefulness of the drug and its tolerability. It is suggested that parenteral administration of antipsychotics would bypass the gut and be more successful in overcoming TRS. The composition of gut microbiota is variable between individuals, but they are seen to remain stable. However, phyla can change in response to many factors including ageing, diet, substance-use, and medications – especially antibiotics, laxatives, and antipsychotics. In FEP, schizophrenia has been linked to significant changes in the gut microbiota that can predict response to treatment.[200]
Psychosocial interventions
A number of psychosocial interventions that include several types of psychotherapy may be useful in the treatment of schizophrenia such as: family therapy,[201] group therapy, cognitive remediation therapy,[202] cognitive behavioral therapy, and metacognitive training.[203] Skills training, and help with substance use, and weight management– often needed as a side effect of an antipsychotic, are also offered.[204] In the US, interventions for first episode psychosis have been brought together in an overall approach known as coordinated speciality care (CSC) and also includes support for education.[30] In the UK care across all phases is a similar approach that covers many of the treatment guidelines recommended.[28] The aim is to reduce the number of relapses and stays in hospital.[201]
Other support services for education, employment, and housing are usually offered. For people suffering from severe schizophrenia, and discharged from a stay in hospital, these services are often brought together in an integrated approach to offer support in the community away from the hospital setting. In addition to medicine management, housing, and finances, assistance is given for more routine matters such as help with shopping and using public transport. This approach is known as assertive community treatment (ACT) and has been shown to achieve positive results in symptoms, social functioning and quality of life.[205][206] Another more intense approach is known as intensive care management (ICM). ICM is a stage further than ACT and emphasises support of high intensity in smaller caseloads, (less than twenty). This approach is to provide long-term care in the community. Studies show that ICM improves many of the relevant outcomes including social functioning.[207]
Some studies have shown little evidence for the effectiveness of cognitive behavioral therapy (CBT) in either reducing symptoms or preventing relapse.[208][209] However, other studies have found that CBT does improve overall psychotic symptoms (when in use with medication) and has been recommended in Canada, but it has been seen here to have no effect on social function, relapse, or quality of life.[210] In the UK it is recommended as an add-on therapy in the treatment of schizophrenia, but is not supported for use in treatment resistant schizophrenia.[209][211] Arts therapies are seen to improve negative symptoms in some people, and are recommended by NICE in the UK.[163][212] This approach however, is criticised as having not been well-researched, and arts therapies are not recommended in Australian guidelines for example.[212][213][214] Peer support, in which people with personal experience of schizophrenia, provide help to each other, is of unclear benefit.[215]
Other
Exercise therapy has been shown to improve positive and negative symptoms, cognition, and improve quality of life.[216] Aerobic exercise has been shown to improve cognitive deficits of working memory and attention.[217] Exercise has also been shown to increase the volume of the hippocampus in those with schizophrenia. A decrease in hippocampal volume is one of the factors linked to the development of the disease.[216] However, there still remains the problem of increasing motivation for, and maintaining participation in physical activity.[218] Supervised sessions are recommended.[217] In the UK healthy eating advice is offered alongside exercise programs.[219]
An inadequate diet is often found in schizophrenia, and associated vitamin deficiencies including those of folate, and vitamin D are linked to the risk factors for the development of schizophrenia and for early death including heart disease.[220] Those with schizophrenia possibly have the worst diet of all the mental disorders. Lower levels of folate and vitamin D have been noted as significantly lower in first episode psychosis.[220] The use of supplemental folate is recommended.[221] A zinc deficiency has also been noted.[222] Vitamin B12 is also often deficient and this is linked to worse symptoms. Supplementation with B vitamins has been shown to significantly improve symptoms, and to put in reverse some of the cognitive deficits.[220] It is also suggested that the noted dysfunction in gut microbiota might benefit from the use of probiotics.[222]
Prognosis
|
no data
≤ 185
185–197
197–207
207–218
218–229
229–240
|
240–251
251–262
262–273
273–284
284–295
≥ 295
|
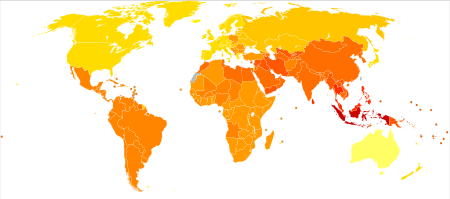
Schizophrenia has great human and economic costs.[5] It results in a decreased life expectancy of 20 years.[10][4] This is primarily because of its association with obesity, poor diet, a sedentary lifestyle, and smoking, with an increased rate of suicide playing a lesser role.[10][223] Side effects of antipsychotics may also increase the risk.[10] These differences in life expectancy increased between the 1970s and 1990s.[224] An Australian study puts the rate of early death at 25 years, and views the main cause to be related to heart disease.[185] Primary polydipsia, or excessive fluid intake, is relatively common in people with chronic schizophrenia.[225][226] This may lead to hyponatremia which can be life-threatening. Antipsychotics can lead to a dry mouth, but there are several other factors that may contribute to the disorder. It is suggested to lead to a reduction in life expectancy by 13 per cent.[226] A study has suggested that real barriers to improving the mortality rate in schizophrenia are poverty, overlooking the symptoms of other illnesses, stress, stigma, and medication side effects, and that these need to be changed.[227]
Schizophrenia is a major cause of disability. In 2016 it was classed as the 12th most disabling condition.[228] Approximately 75% of people with schizophrenia have ongoing disability with relapses[40] and 16.7 million people globally are deemed to have moderate or severe disability from the condition.[229] Some people do recover completely and others function well in society.[230] Most people with schizophrenia live independently with community support.[22] About 85% are unemployed.[5] In people with a first episode of psychosis in scizophrenia a good long-term outcome occurs in 31%, an intermediate outcome in 42% and a poor outcome in 31%.[231] Males are affected more often than females, and have a worse outcome.[232] Outcomes for schizophrenia appear better in the developing than the developed world.[233] These conclusions have been questioned.[234] Social problems, such as long-term unemployment, poverty, homelessness, exploitation, stigmatization and victimization are common consequences, and lead to social exclusion.[20][21]
There is a higher than average suicide rate associated with schizophrenia estimated at around 5% to 6%, most often occurring in the period following onset or first hospital admission.[23][11] Several times more (20 to 40%) attempt suicide at least once.[7][235] There are a variety of risk factors, including male gender, depression, a high IQ,[235] heavy smoking,[236] and substance abuse.[100] Repeated relapse is linked to an increased risk of suicidal behavior.[158] The use of clozapine can reduce the risk of suicide and aggression.[188]
Schizophrenia and smoking have shown a strong association in studies worldwide.[237][238] Use of cigarettes is especially high in those diagnosed with schizophrenia, with estimates ranging from 80 to 90% being regular smokers, as compared to 20% of the general population.[238] Those who smoke tend to smoke heavily, and additionally smoke cigarettes with high nicotine content.[33] Some propose that this is in an effort to improve symptoms.[239] Among people with schizophrenia use of cannabis is also common.[100]
Epidemiology
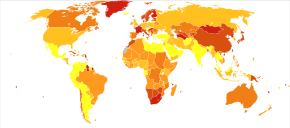
In 2017, the Global Burden of Disease Study estimated there were 1.1 million new cases, and in 2019 WHO reported a total of 20 million cases globally.[15][2] Schizophrenia affects around 0.3–0.7% of people at some point in their life.[14] It occurs 1.4 times more frequently in males than females and typically appears earlier in men[72] – the peak ages of onset are 25 years for males and 27 years for females.[240] Onset in childhood, before the age of 13 can sometimes occur.[7][59] A later onset can occur between the ages of 40 and 60, known as late onset, and also after 60 known as very late onset.[50]
Worldwide, schizophrenia is the most common psychotic disorder.[62] The frequency of schizophrenia varies across the world,[7][241] within countries,[242] and at the local and neighborhood level.[243] This variation has been estimated to be fivefold.[5] It causes approximately one percent of worldwide disability adjusted life years[72] and resulted in 17,000 deaths in 2015.[12]
In 2000, the World Health Organization found the percentage of people affected and the number of new cases that develop each year is roughly similar around the world, with age-standardized prevalence per 100,000 ranging from 343 in Africa to 544 in Japan and Oceania for men, and from 378 in Africa to 527 in Southeastern Europe for women.[244] About 1.1% of adults have schizophrenia in the United States.[245] However, in areas of conflict this figure can rise to between 4.0 and 6.5%.[246]
History

The history of schizophrenia is complex and does not lend itself easily to a linear narrative.[247] Accounts of a schizophrenia-like syndrome are rare in records before the 19th century. The earliest cases detailed were reported in 1797, and 1809.[248] Dementia praecox, meaning premature dementia was used by German psychiatrist Heinrich Schüle in 1886, and then in 1891 by Arnold Pick in a case report of hebephrenia. In 1893 Emil Kraepelin used the term in making a distinction, known as the Kraepelinian dichotomy, between the two psychoses – dementia praecox, and manic depression (now called bipolar disorder).[10] Kraepelin believed that dementia praecox was probably caused by a systemic disease that affected many organs and nerves, affecting the brain after puberty in a final decisive cascade.[249] It was thought to be an early form of dementia, a degenerative disease.[10] When it became evident that the disorder was not degenerative it was renamed schizophrenia by Eugen Bleuler in 1908.[250]
The word schizophrenia translates roughly as "splitting of the mind" and is Modern Latin from the Greek roots schizein (σχίζειν, "to split") and phrēn, (φρεν, "mind")[251] Its use was intended to describe the separation of function between personality, thinking, memory, and perception.[250]
The term schizophrenia used to be associated with split personality by the general population but that usage went into decline when split personality became known as a separate disorder, first as multiple identity disorder , and later as dissociative identity disorder.[252] In 2002 in Japan the name was changed to integration disorder, and in 2012 in South Korea, the name was changed to attunement disorder to reduce the stigma, both with good results.[22][253][254]
In the early 20th century, the psychiatrist Kurt Schneider listed the psychotic symptoms of schizophrenia into two groups of hallucinations, and delusions. The hallucinations were listed as specific to auditory, and the delusional included thought disorders. These were seen as the symptoms of first-rank importance and were termed first-rank symptoms. Whilst these were also sometimes seen to be relevant to the psychosis in manic-depression, they were highly suggestive of schizophrenia and typically referred to as first-rank symptoms of schizophrenia. The most common first-rank symptom was found to belong to thought disorders.[255][256] In 2013 the first-rank symptoms were excluded from the DSM-5 criteria.[146] First-rank symptoms are seen to be of limited use in detecting schizophrenia but may be of help in differential diagnosis.[257]
The earliest attempts to treat schizophrenia were psychosurgical, involving either the removal of brain tissue from different regions or the severing of pathways.[258] These were notably frontal lobotomies and cingulotomies which were carried out from the 1930s.[258][259] In the 1930s a number of shock therapies were introduced which induced seizures (convulsions) or comas.[260] Insulin shock therapy involved the injecting of large doses of insulin in order to induce comas, which in turn produced hypoglycemia and convulsions.[260][259] The use of electricity to induce seizures was developed, and in use as electroconvulsive therapy (ECT) by 1938.[261] Stereotactic surgeries were developed in the 1940s.[261] Treatment was revolutionized in the mid-1950s with the development and introduction of the first typical antipsychotic, chlorpromazine.[262] In the 1970s the first atypical antipsychotic clozapine, was introduced followed by the introduction of others.[263]
In the early 1970s in the US, the diagnostic model used for schizophrenia was broad and clinically-based using DSM II. It had been noted that schizophrenia was diagnosed far more in the US than in Europe which had been using the ICD-9 criteria. The US model was criticised for failing to demarcate clearly those people with a mental illness, and those without. In 1980 DSM III was published and showed a shift in focus from the clinically-based biopsychosocial model to a reason-based medical model.[264] DSM IV showed an increased focus to an evidence-based medical model.[265]
Subtypes of schizophrenia are no longer recognized as separate conditions from schizophrenia by DSM-5[266] or ICD-11.[267] Before 2013, the subtypes of schizophrenia were classified as paranoid, disorganized, catatonic, undifferentiated, and residual type.[268] The subtypes of schizophrenia were eliminated because of a lack of clear distinction among the subtypes and low validity of classification.[267][269]
Society and culture

In 2002, the term for schizophrenia in Japan was changed from seishin-bunretsu-byō (精神分裂病, lit. "mind-split disease") to tōgō-shitchō-shō (統合失調症, lit. "integration-dysregulation syndrome") to reduce stigma.[270] The new name also interpreted as "integration disorder" was inspired by the biopsychosocial model; it increased the percentage of people who were informed of the diagnosis from 37 to 70% over three years.[253] A similar change was made in South Korea in 2012 to attunement disorder.[254] A professor of psychiatry, Jim van Os, has proposed changing the English term to psychosis spectrum syndrome.[271] In 2013 with the reviewed DSM-5, the DSM-5 committee was in favor of giving a new name to schizophrenia but they referred this to WHO.[272]
In the United States, the cost of schizophrenia – including direct costs (outpatient, inpatient, drugs, and long-term care) and non-health care costs (law enforcement, reduced workplace productivity, and unemployment) – was estimated to be $62.7 billion in 2002.[273] In the UK the cost in 2016 was put at £11.8 billion per year with a third of that figure directly attributable to the cost of hospital and social care, and treatment.[5]
The book A Beautiful Mind chronicled the life of John Forbes Nash who had been diagnosed with schizophrenia but who went on to win the Nobel Prize for Economics. This was later made into the film with the same name. An earlier documentary was made with the title A Brilliant Madness.
In 1964 a lengthy case study of three males diagnosed with paranoid schizophrenia who each had the delusional belief that they were Jesus Christ was published as a book. This has the title of The Three Christs of Ypsilanti, and a film with the title Three Christs was released in 2020. Such religious delusions are a fairly common feature in psychoses including schizophrenia.[274][275]
Violence
People with severe mental illness, including schizophrenia, are at a significantly greater risk of being victims of both violent and non-violent crime.[276] Schizophrenia has been associated with a higher rate of violent acts, but most appear to be related to associated substance abuse.[277] Rates of homicide linked to psychosis are similar to those linked to substance misuse, and parallel the overall rate in a region.[278] What role schizophrenia has on violence independent of drug misuse is controversial, but certain aspects of individual histories or mental states may be factors.[279] About 11% of people in prison for homicide have schizophrenia and 21% have mood disorders.[280] Another study found about 8-10% of people with schizophrenia had committed a violent act in the past year compared to 2% of the general population.[280]
Media coverage relating to violent acts by people with schizophrenia reinforces public perception of an association between schizophrenia and violence.[277] In a large, representative sample from a 1999 study, 12.8% of Americans believed that those with schizophrenia were "very likely" to do something violent against others, and 48.1% said that they were "somewhat likely" to. Over 74% said that people with schizophrenia were either "not very able" or "not able at all" to make decisions concerning their treatment, and 70.2% said the same of money-management decisions.[281] The perception of people with psychosis as violent more than doubled between the 1950s and 2000, according to one meta-analysis.[282]
Research directions
Schizophrenia is not believed to occur in other animals[283] but it may be possible to develop a pharmacologically induced non-human primate model of schizophrenia.[284]
Effects of early intervention is an active area of research.[156] One important aspect of this research is early detection of at-risk individuals. This includes development of risk calculators[285] and methods for large-scale population screening.[286]
Various agents have been explored for possible effectiveness in treating negative symptoms, for which antipsychotics have been of little benefit.[287] There have been trials on medications with anti-inflammatory activity, based on the premise that inflammation might play a role in the pathology of schizophrenia.[288]
Research has found a tentative benefit in using minocycline, a broad-spectrum antibiotic, as an add-on treatment for schizophrenia. Reviews have found that minocycline as an add-on therapy appears to be effective in improving all dimensions of symptoms, and has been found to be safe and well tolerated, but larger studies are called for.[289][290]
A review of the effects of nidotherapy – efforts to change the environment to improve functional ability was inconclusive, and it was suggested that it be treated as an experimental approach.[291]
Various brain stimulation techniques are being studied to treat the positive symptoms of schizophrenia, in particular auditory verbal hallucinations (AVHs).[292][293] A 2015 Cochrane review found unclear evidence of benefit.[294] Most studies focus on transcranial direct-current stimulation (tDCM), and repetitive transcranial magnetic stimulation (rTMS).[293] Techniques based on focused ultrasound for deep brain stimulation could provide insight for the treatment of AVHs.[293]
Another active area of research is the study of a variety of potential biomarkers that would be of invaluable help not only in the diagnosis but also in the treatment and prognosis of schizophrenia. Possible biomarkers include markers of inflammation, neuroimaging, BDNF, genetics, and speech analysis. Some inflammatory markers such as C-reactive protein are useful in detecting levels of inflammation implicated in some psychiatric disorders but they are not disorder-specific. However, other inflammatory cytokines are found to be elevated in first episode psychosis and acute relapse that are normalized after treatment with antipsychotics, and these may be considered as state markers.[295] Deficits in sleep spindles in schizophrenia may serve as a marker of an impaired thalamocortical circuit, and a mechanism for memory impairment.[149]
References
- Jones D (2003) [1917]. Roach P, Hartmann J, Setter J (eds.). English Pronouncing Dictionary. Cambridge: Cambridge University Press. ISBN 978-3-12-539683-8.
- "Schizophrenia Fact sheet". www.who.int. 4 October 2019. Retrieved 22 January 2020.
- "Schizophrenia". National Institute of Mental Health. January 2016. Archived from the original on 25 November 2016. Retrieved 3 February 2016.
- "Medicinal treatment of psychosis/schizophrenia". www.sbu.se. Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). 21 November 2012. Archived from the original on 29 June 2017. Retrieved 26 June 2017.
- Owen MJ, Sawa A, Mortensen PB (July 2016). "Schizophrenia". Lancet. 388 (10039): 86–97. doi:10.1016/S0140-6736(15)01121-6. PMC 4940219. PMID 26777917.
- Gruebner O, Rapp MA, Adli M, et al. (February 2017). "Cities and mental health". Deutsches Ärzteblatt International. 114 (8): 121–127. doi:10.3238/arztebl.2017.0121. PMC 5374256. PMID 28302261.
- Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). American Psychiatric Association. 2013. pp. 99–105. ISBN 978-0-89042-555-8.
- Ferri FF (2010). Ferri's differential diagnosis : a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter S. ISBN 978-0-323-07699-9.
- Paris J (December 2018). "Differential Diagnosis of Borderline Personality Disorder". The Psychiatric Clinics of North America. 41 (4): 575–582. doi:10.1016/j.psc.2018.07.001. PMID 30447725.
- Laursen TM, Nordentoft M, Mortensen PB (2014). "Excess early mortality in schizophrenia". Annual Review of Clinical Psychology. 10: 425–48. doi:10.1146/annurev-clinpsy-032813-153657. PMID 24313570.
- Ferri, Fred F. (2019). Ferri's clinical advisor 2019 : 5 books in 1. pp. 1225–1226. ISBN 9780323530422.
- GBD 2015 Mortality and Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- "Types of psychosis". www.mind.org.uk. Retrieved 25 January 2020.
- Javitt DC (June 2014). "Balancing therapeutic safety and efficacy to improve clinical and economic outcomes in schizophrenia: a clinical overview". The American Journal of Managed Care. 20 (8 Suppl): S160-5. PMID 25180705.
- James SL, Abate D (November 2018). "Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017". The Lancet. 392 (10159): 1789–1858. doi:10.1016/S0140-6736(18)32279-7. PMC 6227754. PMID 30496104.
- van de Leemput J, Hess JL, Glatt SJ, Tsuang MT (2016). "Genetics of Schizophrenia: Historical Insights and Prevailing Evidence". Advances in Genetics. 96: 99–141. doi:10.1016/bs.adgen.2016.08.001. PMID 27968732.
- Parakh P, Basu D (August 2013). "Cannabis and psychosis: have we found the missing links?". Asian Journal of Psychiatry (Review). 6 (4): 281–7. doi:10.1016/j.ajp.2013.03.012. PMID 23810133.
Cannabis acts as a component cause of psychosis, that is, it increases the risk of psychosis in people with certain genetic or environmental vulnerabilities, though by itself, it is neither a sufficient nor a necessary cause of psychosis.
- Vita A, Barlati S (May 2018). "Recovery from schizophrenia: is it possible?". Current Opinion in Psychiatry. 31 (3): 246–255. doi:10.1097/YCO.0000000000000407. PMID 29474266. S2CID 35299996.
- Lawrence RE, First MB, Lieberman JA (2015). "Chapter 48: Schizophrenia and Other Psychoses". In Tasman A, Kay J, Lieberman JA, First MB, Riba MB (eds.). Psychiatry (fourth ed.). John Wiley & Sons, Ltd. pp. 798, 816, 819. doi:10.1002/9781118753378.ch48. ISBN 978-1-118-84547-9.
- Killaspy H (September 2014). "Contemporary mental health rehabilitation". East Asian Archives of Psychiatry. 24 (3): 89–94. PMID 25316799.
- Charlson FJ, Ferrari AJ, Santomauro DF, et al. (17 October 2018). "Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016". Schizophrenia Bulletin. 44 (6): 1195–1203. doi:10.1093/schbul/sby058. PMC 6192504. PMID 29762765.
- van Os J, Kapur S (August 2009). "Schizophrenia" (PDF). Lancet. 374 (9690): 635–45. doi:10.1016/S0140-6736(09)60995-8. PMID 19700006. S2CID 208792724. Archived from the original (PDF) on 23 June 2013. Retrieved 23 December 2011.
- Hor K, Taylor M (November 2010). "Suicide and schizophrenia: a systematic review of rates and risk factors". Journal of Psychopharmacology. 24 (4 Suppl): 81–90. doi:10.1177/1359786810385490. PMC 2951591. PMID 20923923.
- Siskind D, Siskind V, Kisely S (November 2017). "Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis". Canadian Journal of Psychiatry. 62 (11): 772–777. doi:10.1177/0706743717718167. PMC 5697625. PMID 28655284.
- Becker T, Kilian R (2006). "Psychiatric services for people with severe mental illness across western Europe: what can be generalized from current knowledge about differences in provision, costs and outcomes of mental health care?". Acta Psychiatrica Scandinavica. Supplementum. 113 (429): 9–16. doi:10.1111/j.1600-0447.2005.00711.x. PMID 16445476.
- Capdevielle D, Boulenger JP, Villebrun D, Ritchie K (September 2009). "[Schizophrenic patients' length of stay: mental health care implication and medicoeconomic consequences]". Encephale (in French). 35 (4): 394–9. doi:10.1016/j.encep.2008.11.005. PMID 19748377.
- Narayan KK, Kumar DS (January 2012). "Disability in a Group of Long-stay Patients with Schizophrenia: Experience from a Mental Hospital". Indian Journal of Psychological Medicine. 34 (1): 70–5. doi:10.4103/0253-7176.96164. PMC 3361848. PMID 22661812.
- "Psychosis and schizophrenia in adults: treatment and management" (PDF). NICE. March 2014. pp. 4–34. Archived from the original (PDF) on 20 April 2014. Retrieved 19 April 2014.
- Stępnicki P, Kondej M, Kaczor AA (20 August 2018). "Current Concepts and Treatments of Schizophrenia". Molecules. 23 (8): 2087. doi:10.3390/molecules23082087. PMC 6222385. PMID 30127324.
- "NIMH » RAISE Questions and Answers". www.nimh.nih.gov. Retrieved 29 December 2019.
- Marshall M (September 2005). "Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review". Archives of General Psychiatry. 62 (9): 975–83. doi:10.1001/archpsyc.62.9.975. PMID 16143729.
- Császár N, Kapócs G, Bókkon I (27 May 2019). "A possible key role of vision in the development of schizophrenia". Reviews in the Neurosciences. 30 (4): 359–379. doi:10.1515/revneuro-2018-0022. PMID 30244235. S2CID 52813070.
- American Psychiatric Association. Task Force on DSM-IV. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Pub. pp. 299–304. ISBN 978-0-89042-025-6.
- Heinz A, Voss M, Lawrie SM, et al. (September 2016). "Shall we really say goodbye to first rank symptoms?". European Psychiatry. 37: 8–13. doi:10.1016/j.eurpsy.2016.04.010. PMID 27429167.
- "National Institute of Mental Health". Archived from the original on 28 September 2011. Retrieved 2 October 2011.
- Carson VB (2000). Mental Health Nursing: The Nurse-patient Journey. W.B. Saunders. p. 638. ISBN 978-0-7216-8053-8. Archived from the original on 13 May 2016.
- Adida M, Azorin JM, Belzeaux R, Fakra E (December 2015). "[Negative symptoms: clinical and psychometric aspects]". Encephale (in French). 41 (6 Suppl 1): 6S15–7. doi:10.1016/S0013-7006(16)30004-5. PMID 26776385.
- Hirsch SR, Weinberger DR (2003). Schizophrenia. Wiley-Blackwell. p. 481. ISBN 978-0-632-06388-8.
- Velligan DI, Alphs LD (1 March 2008). "Negative Symptoms in Schizophrenia: The Importance of Identification and Treatment". Psychiatric Times. 25 (3). Archived from the original on 6 October 2009.
- Smith T, Weston C, Lieberman J (August 2010). "Schizophrenia (maintenance treatment)". American Family Physician. 82 (4): 338–9. PMID 20704164.
- Marder SR, Kirkpatrick B (May 2014). "Defining and measuring negative symptoms of schizophrenia in clinical trials". European Neuropsychopharmacology. 24 (5): 737–43. doi:10.1016/j.euroneuro.2013.10.016. PMID 24275698. S2CID 5172022.
- Bozikas VP, Andreou C (February 2011). "Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature". The Australian and New Zealand Journal of Psychiatry. 45 (2): 93–108. doi:10.3109/00048674.2010.541418. PMID 21320033. S2CID 26135485.
- Shah JN, Qureshi SU, Jawaid A, Schulz PE (June 2012). "Is there evidence for late cognitive decline in chronic schizophrenia?". The Psychiatric Quarterly. 83 (2): 127–44. doi:10.1007/s11126-011-9189-8. PMID 21863346. S2CID 10970088.
- Biedermann F, Fleischhacker WW (August 2016). "Psychotic disorders in DSM-5 and ICD-11". CNS Spectrums. 21 (4): 349–54. doi:10.1017/S1092852916000316. PMID 27418328.
- Vidailhet P (September 2013). "[First-episode psychosis, cognitive difficulties and remediation]". L'Encephale. 39 Suppl 2: S83-92. doi:10.1016/S0013-7006(13)70101-5. PMID 24084427.
- Hashimoto K (5 July 2019). "Recent Advances in the Early Intervention in Schizophrenia: Future Direction from Preclinical Findings". Current Psychiatry Reports. 21 (8): 75. doi:10.1007/s11920-019-1063-7. PMID 31278495. S2CID 195814019.
- Green MF, Horan WP, Lee J (June 2019). "Nonsocial and social cognition in schizophrenia: current evidence and future directions". World Psychiatry. 18 (2): 146–161. doi:10.1002/wps.20624. PMC 6502429. PMID 31059632.
- Javitt DC, Sweet RA (September 2015). "Auditory dysfunction in schizophrenia: integrating clinical and basic features". Nature Reviews. Neuroscience. 16 (9): 535–50. doi:10.1038/nrn4002. PMC 4692466. PMID 26289573.
- Megreya AM (2016). "Face perception in schizophrenia: a specific deficit". Cognitive Neuropsychiatry. 21 (1): 60–72. doi:10.1080/13546805.2015.1133407. PMID 26816133. S2CID 26125559.
- Murante T, Cohen CI (January 2017). "Cognitive Functioning in Older Adults With Schizophrenia". Focus (American Psychiatric Publishing). 15 (1): 26–34. doi:10.1176/appi.focus.20160032. PMC 6519630. PMID 31975837.
- Eack SM (July 2012). "Cognitive remediation: a new generation of psychosocial interventions for people with schizophrenia". Social Work. 57 (3): 235–46. doi:10.1093/sw/sws008. PMC 3683242. PMID 23252315.
- Pomarol-Clotet E, Oh M, Laws KR, McKenna PJ (February 2008). "Semantic priming in schizophrenia: systematic review and meta-analysis". The British Journal of Psychiatry. 192 (2): 92–7. doi:10.1192/bjp.bp.106.032102. hdl:2299/2735. PMID 18245021.
- Goldberg TE, Keefe RS, Goldman RS, Robinson DG, Harvey PD (April 2010). "Circumstances under which practice does not make perfect: a review of the practice effect literature in schizophrenia and its relevance to clinical treatment studies". Neuropsychopharmacology. 35 (5): 1053–62. doi:10.1038/npp.2009.211. PMC 3055399. PMID 20090669.
- King DJ, Hodgekins J, Chouinard PA, Chouinard VA, Sperandio I (June 2017). "A review of abnormalities in the perception of visual illusions in schizophrenia". Psychonomic Bulletin and Review. 24 (3): 734–751. doi:10.3758/s13423-016-1168-5. PMC 5486866. PMID 27730532.
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ (September 2010). "Facial emotion perception in schizophrenia: a meta-analytic review". Schizophrenia Bulletin. 36 (5): 1009–19. doi:10.1093/schbul/sbn192. PMC 2930336. PMID 19329561. Archived from the original on 25 July 2015.
- Le Gall, E; Iakimova, G (December 2018). "[Social cognition in schizophrenia and autism spectrum disorder: Points of convergence and functional differences]". L'Encephale. 44 (6): 523–537. doi:10.1016/j.encep.2018.03.004. PMID 30122298.
- Grill-Spector K, Weiner KS, Kay K, Gomez J (15 September 2017). "The Functional Neuroanatomy of Human Face Perception". Annual Review of Vision Science. 3: 167–196. doi:10.1146/annurev-vision-102016-061214. PMC 6345578. PMID 28715955.
- Bourgou Gaha S, Halayem Dhouib S, Amado I, Bouden A (June 2015). "[Neurological soft signs in early onset schizophrenia]". L'Encephale. 41 (3): 209–14. doi:10.1016/j.encep.2014.01.005. PMID 24854724.
- Da Fonseca D, Fourneret P (December 2018). "[Very early onset schizophrenia]". L'Encephale. 44 (6S): S8–S11. doi:10.1016/S0013-7006(19)30071-5. PMID 30935493.
- Häfner H (2019). "From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness". Psychiatry Journal. 2019: 9804836. doi:10.1155/2019/9804836. PMC 6500669. PMID 31139639.
- Cohen CI, Freeman K, Ghoneim D, et al. (March 2018). "Advances in the Conceptualization and Study of Schizophrenia in Later Life". The Psychiatric Clinics of North America. 41 (1): 39–53. doi:10.1016/j.psc.2017.10.004. PMID 29412847.
- Kar SK, Jain M (July 2016). "Current understandings about cognition and the neurobiological correlates in schizophrenia". Journal of Neurosciences in Rural Practice. 7 (3): 412–8. doi:10.4103/0976-3147.176185. PMC 4898111. PMID 27365960.
- George M, Maheshwari S, Chandran S, Manohar JS, Sathyanarayana Rao TS (October 2017). "Understanding the schizophrenia prodrome". Indian Journal of Psychiatry. 59 (4): 505–509. doi:10.4103/psychiatry.IndianJPsychiatry_464_17 (inactive 3 August 2020). PMC 5806335. PMID 29497198.
- Conroy S, Francis M, Hulvershorn LA (March 2018). "Identifying and treating the prodromal phases of bipolar disorder and schizophrenia". Current Treatment Options in Psychiatry. 5 (1): 113–128. doi:10.1007/s40501-018-0138-0. PMC 6196741. PMID 30364516.
- Fountoulakis KN, Panagiotidis P, Kimiskidis V, Nimatoudis I, Gonda X (February 2019). "Neurological soft signs in familial and sporadic schizophrenia". Psychiatry Research. 272: 222–229. doi:10.1016/j.psychres.2018.12.105. PMID 30590276. S2CID 56476015.
- Davis J, Eyre H, Jacka FN, et al. (June 2016). "A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis". Neurosci Biobehav Rev. 65: 185–94. doi:10.1016/j.neubiorev.2016.03.017. PMC 4876729. PMID 27073049.
- Perkovic MN, Erjavec GN, Strac DS, et al. (March 2017). "Theranostic biomarkers for schizophrenia". International Journal of Molecular Sciences. 18 (4): 733. doi:10.3390/ijms18040733. PMC 5412319. PMID 28358316.
- Mullin AP, Gokhale A, Moreno-De-Luca A, Sanyal S, Waddington JL, Faundez V (December 2013). "Neurodevelopmental disorders: mechanisms and boundary definitions from genomes, interactomes and proteomes". Transl Psychiatry. 3 (12): e329. doi:10.1038/tp.2013.108. PMC 4030327. PMID 24301647.
- Combs DR, Mueser KT, Gutierrez MM (2011). "Chapter 8: Schizophrenia: Etiological considerations". In Hersen M, Beidel DC (eds.). Adult psychopathology and diagnosis (6th ed.). John Wiley & Sons. ISBN 978-1-118-13884-7.
- O'Donovan MC, Williams NM, Owen MJ (October 2003). "Recent advances in the genetics of schizophrenia". Human Molecular Genetics. 12 Spec No 2: R125–33. doi:10.1093/hmg/ddg302. PMID 12952866.
- Torrey EF, Yolken RH (August 2019). "Schizophrenia as a pseudogenetic disease: A call for more gene-environmental studies". Psychiatry Research. 278: 146–150. doi:10.1016/j.psychres.2019.06.006. PMID 31200193. S2CID 173991937.
- Picchioni MM, Murray RM (July 2007). "Schizophrenia". BMJ. 335 (7610): 91–5. doi:10.1136/bmj.39227.616447.BE. PMC 1914490. PMID 17626963.
- Farrell MS, Werge T, Sklar P, et al. (May 2015). "Evaluating historical candidate genes for schizophrenia". Molecular Psychiatry. 20 (5): 555–62. doi:10.1038/mp.2015.16. PMC 4414705. PMID 25754081.
- Schulz, S. Charles; Green, Michael Foster; Nelson, Katharine J. (2016). Schizophrenia and Psychotic Spectrum Disorders. Oxford University Press. pp. 124–5. ISBN 9780199378067.
- Schork AJ, Wang Y, Thompson WK, Dale AM, Andreassen OA (February 2016). "New statistical approaches exploit the polygenic architecture of schizophrenia—implications for the underlying neurobiology". Current Opinion in Neurobiology. 36: 89–98. doi:10.1016/j.conb.2015.10.008. PMC 5380793. PMID 26555806.
- Kendler KS (March 2016). "The Schizophrenia Polygenic Risk Score: To What Does It Predispose in Adolescence?". JAMA Psychiatry. 73 (3): 193–4. doi:10.1001/jamapsychiatry.2015.2964. PMID 26817666.
- Lowther C, Costain G, Baribeau DA, Bassett AS (September 2017). "Genomic Disorders in Psychiatry-What Does the Clinician Need to Know?". Current Psychiatry Reports. 19 (11): 82. doi:10.1007/s11920-017-0831-5. PMID 28929285. S2CID 4776174.
- Bundy H, Stahl D, MacCabe JH (February 2011). "A systematic review and meta-analysis of the fertility of patients with schizophrenia and their unaffected relatives: Fertility in schizophrenia". Acta Psychiatrica Scandinavica. 123 (2): 98–106. doi:10.1111/j.1600-0447.2010.01623.x. PMID 20958271.
- van Dongen J, Boomsma DI (March 2013). "The evolutionary paradox and the missing heritability of schizophrenia". American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 162 (2): 122–136. doi:10.1002/ajmg.b.32135. PMID 23355297.
- Owen MJ, Sawa A, Mortensen PB (2 July 2016). "Schizophrenia". Lancet. 388 (10039): 86–97. doi:10.1016/S0140-6736(15)01121-6. PMC 4940219. PMID 26777917.
- Cirulli F, Musillo C, Berry A (5 February 2020). "Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring". Neuroscience. doi:10.1016/j.neuroscience.2020.01.023. PMID 32032668. S2CID 211029692.
- Upthegrove R, Khandaker GM (2020). "Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia" (PDF). Current Topics in Behavioral Neurosciences. 44: 49–66. doi:10.1007/7854_2018_88. ISBN 978-3-030-39140-9. PMID 31115797.
- Chiang M, Natarajan R, Fan X (February 2016). "Vitamin D in schizophrenia: a clinical review". Evidence-based Mental Health. 19 (1): 6–9. doi:10.1136/eb-2015-102117. PMID 26767392. S2CID 206926835.
- Arias I, Sorlozano A, Villegas E, et al. (April 2012). "Infectious agents associated with schizophrenia: a meta-analysis". Schizophrenia Research. 136 (1–3): 128–36. doi:10.1016/j.schres.2011.10.026. PMID 22104141. S2CID 2687441.
- Yolken R (June 2004). "Viruses and schizophrenia: a focus on herpes simplex virus". Herpes. 11 Suppl 2 (Suppl 2): 83A–88A. PMID 15319094.
- Khandaker GM (August 2012). "Childhood infection and adult schizophrenia: a meta-analysis of population-based studies". Schizophr. Res. 139 (1–3): 161–8. doi:10.1016/j.schres.2012.05.023. PMC 3485564. PMID 22704639.
- Pearce J, Murray C, Larkin W (July 2019). "Childhood adversity and trauma:experiences of professionals trained to routinely enquire about childhood adversity". Heliyon. 5 (7): e01900. doi:10.1016/j.heliyon.2019.e01900. PMC 6658729. PMID 31372522.
- Dvir Y, Denietolis B, Frazier JA (October 2013). "Childhood trauma and psychosis". Child and Adolescent Psychiatric Clinics of North America. 22 (4): 629–41. doi:10.1016/j.chc.2013.04.006. PMID 24012077.
- Misiak B, Krefft M, Bielawski T, Moustafa AA, Sąsiadek MM, Frydecka D (April 2017). "Toward a unified theory of childhood trauma and psychosis: A comprehensive review of epidemiological, clinical, neuropsychological and biological findings". Neuroscience and Biobehavioral Reviews. 75: 393–406. doi:10.1016/j.neubiorev.2017.02.015. PMID 28216171. S2CID 21614845.
- Guloksuz S, van Os J (January 2018). "The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum". Psychological Medicine. 48 (2): 229–244. doi:10.1017/S0033291717001775. PMID 28689498.
- CostaESilva JA, Steffen RE (November 2019). "Urban environment and psychiatric disorders: a review of the neuroscience and biology". Metabolism: Clinical and Experimental. 100S: 153940. doi:10.1016/j.metabol.2019.07.004. PMID 31610855.
- van Os J (April 2004). "Does the urban environment cause psychosis?". The British Journal of Psychiatry. 184 (4): 287–8. doi:10.1192/bjp.184.4.287. PMID 15056569.
- Attademo L, Bernardini F, Garinella R, Compton MT (March 2017). "Environmental pollution and risk of psychotic disorders: A review of the science to date". Schizophrenia Research. 181: 55–59. doi:10.1016/j.schres.2016.10.003. PMID 27720315. S2CID 25505446.
- Selten JP, Cantor-Graae E, Kahn RS (March 2007). "Migration and schizophrenia". Current Opinion in Psychiatry. 20 (2): 111–5. doi:10.1097/YCO.0b013e328017f68e. PMID 17278906. S2CID 21391349.
- Sharma R, Agarwal A, Rohra VK, et al. (April 2015). "Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring". Reprod. Biol. Endocrinol. 13: 35. doi:10.1186/s12958-015-0028-x. PMC 4455614. PMID 25928123.
- Gregg L, Barrowclough C, Haddock G (May 2007). "Reasons for increased substance use in psychosis". Clinical Psychology Review. 27 (4): 494–510. doi:10.1016/j.cpr.2006.09.004. PMID 17240501.
- Sagud M, Mihaljević-Peles A, Mück-Seler D, et al. (September 2009). "Smoking and schizophrenia". Psychiatria Danubina. 21 (3): 371–5. PMID 19794359.
- Larson M (30 March 2006). "Alcohol-Related Psychosis". EMedicine. Archived from the original on 9 November 2008. Retrieved 27 September 2006.
- Leweke FM, Koethe D (June 2008). "Cannabis and psychiatric disorders: it is not only addiction". Addiction Biology. 13 (2): 264–75. doi:10.1111/j.1369-1600.2008.00106.x. PMID 18482435.
- Khokhar JY, Henricks AM, Sullivan ED, Green AI (2018). "Unique Effects of Clozapine: A Pharmacological Perspective". Apprentices to Genius: A tribute to Solomon H. Snyder. Advances in Pharmacology (San Diego, Calif.). 82. pp. 137–162. doi:10.1016/bs.apha.2017.09.009. ISBN 9780128140871. PMC 7197512. PMID 29413518.
- Ortiz-Medina MB, Perea M, Torales J, et al. (November 2018). "Cannabis consumption and psychosis or schizophrenia development". The International Journal of Social Psychiatry. 64 (7): 690–704. doi:10.1177/0020764018801690. PMID 30442059. S2CID 53563635.
- "High-strength skunk 'now dominates' UK cannabis market". nhs.uk. 28 February 2018.
- "Schizophrenia". RC Psych Royal College of Psychiatrists. 2020.
- Howes OD (1 January 2017). "The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia". Biological Psychiatry. 81 (1): 9–20. doi:10.1016/j.biopsych.2016.07.014. PMC 5675052. PMID 27720198.
- Broyd A, Balzan RP, Woodward TS, Allen P (June 2017). "Dopamine, cognitive biases and assessment of certainty: A neurocognitive model of delusions". Clinical Psychology Review. 54: 96–106. doi:10.1016/j.cpr.2017.04.006. PMID 28448827.
- Howes OD, Murray RM (May 2014). "Schizophrenia: an integrated sociodevelopmental-cognitive model". Lancet. 383 (9929): 1677–1687. doi:10.1016/S0140-6736(13)62036-X. PMC 4127444. PMID 24315522.
- Grace AA (August 2016). "Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression". Nature Reviews. Neuroscience. 17 (8): 524–32. doi:10.1038/nrn.2016.57. PMC 5166560. PMID 27256556.
- Fusar-Poli P, Meyer-Lindenberg A (January 2013). "Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies". Schizophrenia Bulletin. 39 (1): 33–42. doi:10.1093/schbul/sbr180. PMC 3523905. PMID 22282454.
- Howes OD, Kambeitz J, Kim E, et al. (August 2012). "The nature of dopamine dysfunction in schizophrenia and what this means for treatment". Archives of General Psychiatry. 69 (8): 776–86. doi:10.1001/archgenpsychiatry.2012.169. PMC 3730746. PMID 22474070.
- Arnsten AF, Girgis RR, Gray DL, Mailman RB (January 2017). "Novel Dopamine Therapeutics for Cognitive Deficits in Schizophrenia". Biological Psychiatry. 81 (1): 67–77. doi:10.1016/j.biopsych.2015.12.028. PMC 4949134. PMID 26946382.
- Maia TV, Frank MJ (January 2017). "An Integrative Perspective on the Role of Dopamine in Schizophrenia". Biological Psychiatry. 81 (1): 52–66. doi:10.1016/j.biopsych.2016.05.021. PMC 5486232. PMID 27452791.
- Pratt J, Dawson N, Morris BJ, et al. (February 2017). "Thalamo-cortical communication, glutamatergic neurotransmission and neural oscillations: A unique window into the origins of ScZ?" (PDF). Schizophrenia Research. 180: 4–12. doi:10.1016/j.schres.2016.05.013. PMID 27317361. S2CID 205075178.
- Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV (April 2016). "A quantitative review of the post-mortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits?". Biological Psychology. 116: 57–67. doi:10.1016/j.biopsycho.2015.10.013. PMID 26549579.
- Michie PT, Malmierca MS, Harms L, Todd J (April 2016). "The neurobiology of MMN and implications for schizophrenia". Biological Psychology. 116: 90–7. doi:10.1016/j.biopsycho.2016.01.011. PMID 26826620. S2CID 41179430.
- Marín O (January 2012). "Interneuron dysfunction in psychiatric disorders". Nature Reviews. Neuroscience. 13 (2): 107–20. doi:10.1038/nrn3155. PMID 22251963. S2CID 205507186.
- Lewis DA, Hashimoto T, Volk DW (April 2005). "Cortical inhibitory neurons and schizophrenia". Nature Reviews. Neuroscience. 6 (4): 312–24. doi:10.1038/nrn1648. PMID 15803162. S2CID 3335493.
- Senkowski D, Gallinat J (June 2015). "Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia". Biological Psychiatry. 77 (12): 1010–9. doi:10.1016/j.biopsych.2015.02.034. PMID 25847179. S2CID 206104940.
Several studies that investigated perceptual processes found impaired GBR in ScZ patients over sensory areas, such as the auditory and visual cortex. Moreover, studies examining steady-state auditory-evoked potentials showed deficits in the gen- eration of oscillations in the gamma band.
- Reilly TJ, Nottage JF, Studerus E, et al. (July 2018). "Gamma band oscillations in the early phase of psychosis: A systematic review". Neuroscience and Biobehavioral Reviews. 90: 381–399. doi:10.1016/j.neubiorev.2018.04.006. PMID 29656029. S2CID 4891072.
Decreased gamma power in response to a task was a relatively consistent finding, with 5 out of 6 studies reported reduced evoked or induced power.
- Birnbaum R, Weinberger DR (December 2017). "Genetic insights into the neurodevelopmental origins of schizophrenia". Nature Reviews. Neuroscience. 18 (12): 727–740. doi:10.1038/nrn.2017.125. PMID 29070826. S2CID 3831577.
- Insel TR (November 2010). "Rethinking schizophrenia". Nature. 468 (7321): 187–93. Bibcode:2010Natur.468..187I. doi:10.1038/nature09552. PMID 21068826. S2CID 4416517.
- Khandaker GM, Zimbron J, Lewis G, Jones PB (February 2013). "Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies". Psychological Medicine. 43 (2): 239–57. doi:10.1017/S0033291712000736. PMC 3479084. PMID 22717193.
- Cannon TD (December 2015). "How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis". Trends in Cognitive Sciences. 19 (12): 744–756. doi:10.1016/j.tics.2015.09.009. PMC 4673025. PMID 26493362.
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS (January 2011). "Cognitive control deficits in schizophrenia: mechanisms and meaning". Neuropsychopharmacology. 36 (1): 316–38. doi:10.1038/npp.2010.156. PMC 3052853. PMID 20844478.
- Barch DM, Ceaser A (January 2012). "Cognition in schizophrenia: core psychological and neural mechanisms". Trends in Cognitive Sciences. 16 (1): 27–34. doi:10.1016/j.tics.2011.11.015. PMC 3860986. PMID 22169777.
- Eisenberg DP, Berman KF (January 2010). "Executive function, neural circuitry, and genetic mechanisms in schizophrenia". Neuropsychopharmacology. 35 (1): 258–77. doi:10.1038/npp.2009.111. PMC 2794926. PMID 19693005.
- Walton E, Hibar DP, van Erp TG, et al. (May 2017). "Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium". Acta Psychiatrica Scandinavica. 135 (5): 439–447. doi:10.1111/acps.12718. PMC 5399182. PMID 28369804.
- Walton E, Hibar DP, van Erp TG, et al. (Karolinska Schizophrenia Project Consortium (KaSP)) (January 2018). "Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium". Psychological Medicine. 48 (1): 82–94. doi:10.1017/S0033291717001283. PMC 5826665. PMID 28545597.
- Cohen AS, Minor KS (January 2010). "Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies". Schizophrenia Bulletin. 36 (1): 143–50. doi:10.1093/schbul/sbn061. PMC 2800132. PMID 18562345.
- Strauss GP, Gold JM (April 2012). "A new perspective on anhedonia in schizophrenia". The American Journal of Psychiatry. 169 (4): 364–73. doi:10.1176/appi.ajp.2011.11030447. PMC 3732829. PMID 22407079.
- Young J, Anticevic A, Barch D (2018). "Cognitive and Motivational Neuroscience of Psychotic Disorders". In Charney D, Buxbaum J, Sklar P, Nestler E (eds.). Charney & Nestler's Neurobiology of Mental Illness (5th ed.). New York: Oxford University Press. pp. 215, 217. ISBN 9780190681425.
Several recent reviews (e.g., Cohen and Minor, 2010) have found that individuals with schizophrenia show relatively intact self-reported emotional responses to affect-eliciting stimuli as well as other indicators of intact response(215)...Taken together, the literature increasingly suggests that there may be a deficit in putatively DA-mediated reward learning and/ or reward prediction functions in schizophrenia. Such findings suggest that impairment in striatal reward prediction mechanisms may influence “wanting” in schizophrenia in a way that reduces the ability of individuals with schizophrenia to use anticipated rewards to drive motivated behavior.(217)
- Nemani K, Hosseini Ghomi R, McCormick B, Fan X (January 2015). "Schizophrenia and the gut-brain axis". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 56: 155–60. doi:10.1016/j.pnpbp.2014.08.018. PMID 25240858. S2CID 32315340.
- Lachance LR, McKenzie K (February 2014). "Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis". Schizophrenia Research (Review). 152 (2–3): 521–7. doi:10.1016/j.schres.2013.12.001. PMID 24368154. S2CID 26792210.
- Wiberg A, Ng M, Al Omran Y, et al. (1 October 2019). "Handedness, language areas and neuropsychiatric diseases: insights from brain imaging and genetics". Brain: A Journal of Neurology. 142 (10): 2938–2947. doi:10.1093/brain/awz257. PMC 6763735. PMID 31504236.
- Tzourio-Mazoyer N, Kennedy H, Van Essen DC, Christen Y (2016). "Intra- and Inter-hemispheric Connectivity Supporting Hemispheric Specialization". Micro-, Meso- and Macro-Connectomics of the Brain. Research and Perspectives in Neurosciences. pp. 129–146. doi:10.1007/978-3-319-27777-6_9. ISBN 978-3-319-27776-9. PMID 28590670.
- Ocklenburg S, Güntürkün O, Hugdahl K, Hirnstein M (December 2015). "Laterality and mental disorders in the postgenomic age--A closer look at schizophrenia and language lateralization". Neuroscience and Biobehavioral Reviews. 59: 100–10. doi:10.1016/j.neubiorev.2015.08.019. PMID 26598216. S2CID 15983622.
- Friston KJ, Stephan KE, Montague R, Dolan RJ (July 2014). "Computational psychiatry: the brain as a phantastic organ". The Lancet. Psychiatry. 1 (2): 148–58. doi:10.1016/S2215-0366(14)70275-5. PMID 26360579.
- Griffin JD, Fletcher PC (May 2017). "Predictive Processing, Source Monitoring, and Psychosis". Annual Review of Clinical Psychology. 13: 265–289. doi:10.1146/annurev-clinpsy-032816-045145. PMC 5424073. PMID 28375719.
- Fletcher PC, Frith CD (January 2009). "Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia" (PDF). Nature Reviews. Neuroscience. 10 (1): 48–58. doi:10.1038/nrn2536. PMID 19050712. S2CID 6219485.
- Corlett PR, Taylor JR, Wang XJ, Fletcher PC, Krystal JH (November 2010). "Toward a neurobiology of delusions". Progress in Neurobiology. 92 (3): 345–69. doi:10.1016/j.pneurobio.2010.06.007. PMC 3676875. PMID 20558235.
- Bastos AM, Usrey WM, Adams RA, et al. (21 November 2012). "Canonical microcircuits for predictive coding". Neuron. 76 (4): 695–711. doi:10.1016/j.neuron.2012.10.038. PMC 3777738. PMID 23177956.
- Soltan M, Girguis J (8 May 2017). "How to approach the mental state examination". BMJ. 357: j1821. doi:10.1136/sbmj.j1821. PMID 31055448. Retrieved 9 January 2020.
- Lindenmayer JP (1 December 2017). "Are Shorter Versions of the Positive and Negative Syndrome Scale (PANSS) Doable? A Critical Review". Innovations in Clinical Neuroscience. 14 (11–12): 73–76. PMC 5788254. PMID 29410940.
- Australia, Healthdirect (10 January 2019). "Diagnosis of schizophrenia". www.healthdirect.gov.au. Retrieved 28 January 2020.
- "Schizophrenia - Diagnosis". nhs.uk. 23 October 2017. Retrieved 28 January 2020.
- Jakobsen KD, Frederiksen JN, Hansen T, et al. (2005). "Reliability of clinical ICD-10 schizophrenia diagnoses". Nordic Journal of Psychiatry. 59 (3): 209–12. doi:10.1080/08039480510027698. PMID 16195122. S2CID 24590483.
- Tandon R, Gaebel W, Barch DM, et al. (October 2013). "Definition and description of schizophrenia in the DSM-5". Schizophrenia Research. 150 (1): 3–10. doi:10.1016/j.schres.2013.05.028. PMID 23800613. S2CID 17314600.
- Barch DM, Bustillo J, Gaebel W, et al. (October 2013). "Logic and justification for dimensional assessment of symptoms and related clinical phenomena in psychosis: relevance to DSM-5". Schizophrenia Research. 150 (1): 15–20. doi:10.1016/j.schres.2013.04.027. PMID 23706415. S2CID 10052003.
- Staedt J, Hauser M, Gudlowski Y, Stoppe G (February 2010). "Sleep disorders in schizophrenia". Fortschritte der Neurologie-Psychiatrie. 78 (2): 70–80. doi:10.1055/s-0028-1109967. PMID 20066610.
- Pocivavsek A, Rowland LM (13 January 2018). "Basic Neuroscience Illuminates Causal Relationship Between Sleep and Memory: Translating to Schizophrenia". Schizophrenia Bulletin. 44 (1): 7–14. doi:10.1093/schbul/sbx151. PMC 5768044. PMID 29136236.
- Monti JM, BaHammam AS, Pandi-Perumal SR, et al. (3 June 2013). "Sleep and circadian rhythm dysregulation in schizophrenia". Progress in Neuro-psychopharmacology & Biological Psychiatry. 43: 209–16. doi:10.1016/j.pnpbp.2012.12.021. PMID 23318689. S2CID 19626589.
- Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ (August 2019). "Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis". Sleep Medicine Reviews. 46: 108–123. doi:10.1016/j.smrv.2019.04.012. PMID 31108433.
- Assimakopoulos K, Karaivazoglou K, Skokou M, et al. (24 March 2018). "Genetic Variations Associated with Sleep Disorders in Patients with Schizophrenia: A Systematic Review". Medicines. 5 (2): 27. doi:10.3390/medicines5020027. PMC 6023503. PMID 29587340.
- Bottas A (15 April 2009). "Comorbidity: Schizophrenia With Obsessive-Compulsive Disorder". Psychiatric Times. 26 (4). Archived from the original on 3 April 2013.
- Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's neurology in clinical practice. 1 (6th ed.). Philadelphia, PA: Elsevier/Saunders. pp. 92–111. ISBN 978-1-4377-0434-1.
- Cannon TD, Cornblatt B, McGorry P (May 2007). "The empirical status of the ultra high-risk (prodromal) research paradigm". Schizophrenia Bulletin. 33 (3): 661–4. doi:10.1093/schbul/sbm031. PMC 2526144. PMID 17470445.
- Marshall M, Rathbone J (June 2011). "Early intervention for psychosis". The Cochrane Database of Systematic Reviews (6): CD004718. doi:10.1002/14651858.CD004718.pub3. PMC 4163966. PMID 21678345.
- Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T (January 2013). "Early interventions to prevent psychosis: systematic review and meta-analysis". BMJ. 346: f185. doi:10.1136/bmj.f185. PMC 3548617. PMID 23335473.
- Taylor M, Jauhar S (September 2019). "Are we getting any better at staying better? The long view on relapse and recovery in first episode nonaffective psychosis and schizophrenia". Therapeutic Advances in Psychopharmacology. 9: 204512531987003. doi:10.1177/2045125319870033. PMC 6732843. PMID 31523418.
- Weiden, PJ (July 2016). "Beyond Psychopharmacology: Emerging Psychosocial Interventions for Core Symptoms of Schizophrenia". Focus (American Psychiatric Publishing). 14 (3): 315–327. doi:10.1176/appi.focus.20160014. PMC 6526802. PMID 31975812.
- McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A (March 2007). "Cognitive training for supported employment: 2-3 year outcomes of a randomized controlled trial". The American Journal of Psychiatry. 164 (3): 437–41. doi:10.1176/appi.ajp.164.3.437. PMID 17329468.
- Souaiby L, Gaillard R, Krebs MO (August 2016). "[Duration of untreated psychosis: A state-of-the-art review and critical analysis]". Encephale. 42 (4): 361–6. doi:10.1016/j.encep.2015.09.007. PMID 27161262.
- Fuller Torrey E (June 2015). "Deinstitutionalization and the rise of violence". CNS Spectrums. 20 (3): 207–14. doi:10.1017/S1092852914000753. PMID 25683467.
- "Schizophrenia - Treatment". nhs.uk. 23 October 2017. Retrieved 8 January 2020.
- Ortiz-Orendain, J (12 December 2019). "Modafinil for people with schizophrenia or related disorders". The Cochrane Database of Systematic Reviews. 12: CD008661. doi:10.1002/14651858.CD008661.pub2. PMC 6906203. PMID 31828767.
- Lally J, MacCabe JH (2015). "Antipsychotic medication in schizophrenia: a review". British Medical Bulletin. 114 (1): 169–179. doi:10.1093/bmb/ldv017. PMID 25957394.
- Keks N, Schwartz D, Hope J (October 2019). "Stopping and switching antipsychotic drugs". Australian Prescriber. 42 (5): 152–157. doi:10.18773/austprescr.2019.052. PMC 6787301. PMID 31631928.
- Harrow M, Jobe TH (September 2013). "Does long-term treatment of schizophrenia with antipsychotic medications facilitate recovery?". Schizophrenia Bulletin. 39 (5): 962–5. doi:10.1093/schbul/sbt034. PMC 3756791. PMID 23512950.
- Cather C, Pachas GN, Cieslak KM, Evins AE (June 2017). "Achieving Smoking Cessation in Individuals with Schizophrenia: Special Considerations". CNS Drugs. 31 (6): 471–481. doi:10.1007/s40263-017-0438-8. PMC 5646360. PMID 28550660.
- Tsuda Y, Saruwatari J, Yasui-Furukori N (4 March 2014). "Meta-analysis: the effects of smoking on the disposition of two commonly used antipsychotic agents, olanzapine and clozapine". BMJ Open. 4 (3): e004216. doi:10.1136/bmjopen-2013-004216. PMC 3948577. PMID 24595134.
- Li P, Snyder GL, Vanover KE (2016). "Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future". Current Topics in Medicinal Chemistry. 16 (29): 3385–3403. doi:10.2174/1568026616666160608084834. PMC 5112764. PMID 27291902.
- Lowe EJ, Ackman ML (April 2010). "Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment". The Annals of Pharmacotherapy. 44 (4): 727–32. doi:10.1345/aph.1M398. PMID 20233914. S2CID 11456024.
- Baier M (August 2010). "Insight in schizophrenia: a review". Current Psychiatry Reports. 12 (4): 356–61. doi:10.1007/s11920-010-0125-7. PMID 20526897. S2CID 29323212.
- Peters L, Krogmann A, von Hardenberg L, et al. (19 November 2019). "Long-Acting Injections in Schizophrenia: a 3-Year Update on Randomized Controlled Trials Published January 2016-March 2019". Current Psychiatry Reports. 21 (12): 124. doi:10.1007/s11920-019-1114-0. PMID 31745659. S2CID 208144438.
- Leucht S, Tardy M, Komossa K, et al. (June 2012). "Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis". Lancet. 379 (9831): 2063–71. doi:10.1016/S0140-6736(12)60239-6. PMID 22560607. S2CID 2018124.
- McEvoy JP (2006). "Risks versus benefits of different types of long-acting injectable antipsychotics". The Journal of Clinical Psychiatry. 67 Suppl 5: 15–8. PMID 16822092.
- "FDA Approves Caplyta (lumateperone) for the Treatment of Schizophrenia in Adults". drugs.com. 23 December 2019.
- Blair HA (14 February 2020). "Lumateperone: First Approval". Drugs. 80 (4): 417–423. doi:10.1007/s40265-020-01271-6. PMID 32060882. S2CID 211110160.
- Barry SJ, Gaughan TM, Hunter R (June 2012). "Schizophrenia". BMJ Clinical Evidence. 2012. PMC 3385413. PMID 23870705. Archived from the original on 11 September 2014.
- Ware MR, Feller DB, Hall KL (4 January 2018). "Neuroleptic Malignant Syndrome: Diagnosis and Management". The Primary Care Companion for CNS Disorders. 20 (1). doi:10.4088/PCC.17r02185. PMID 29325237.
- Carbon M, Kane JM, Leucht S, Correll CU (October 2018). "Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis". World Psychiatry. 17 (3): 330–340. doi:10.1002/wps.20579. PMC 6127753. PMID 30192088.
- De Berardis D, Rapini G, Olivieri L, et al. (May 2018). "Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine". Therapeutic Advances in Drug Safety. 9 (5): 237–256. doi:10.1177/2042098618756261. PMC 5956953. PMID 29796248.
- Legge SE, Walters JT (March 2019). "Genetics of clozapine-associated neutropenia: recent advances, challenges and future perspective". Pharmacogenomics. 20 (4): 279–290. doi:10.2217/pgs-2018-0188. PMC 6563116. PMID 30767710.
- Manu P, Lapitskaya Y, Shaikh A, Nielsen J (2018). "Clozapine Rechallenge After Major Adverse Effects: Clinical Guidelines Based on 259 Cases". American Journal of Therapeutics. 25 (2): e218–e223. doi:10.1097/MJT.0000000000000715. PMID 29505490. S2CID 3689529.
- Lally J, McCaffrey C, O Murchu C, et al. (July 2019). "Clozapine Rechallenge Following Neuroleptic Malignant Syndrome: A Systematic Review". Journal of Clinical Psychopharmacology. 39 (4): 372–379. doi:10.1097/JCP.0000000000001048. PMID 31205196. S2CID 189945135.
- Kritharides L, Chow V, Lambert TJ (6 February 2017). "Cardiovascular disease in patients with schizophrenia". The Medical Journal of Australia. 206 (2): 91–95. doi:10.5694/mja16.00650. PMID 28152356.
- Sarvaiya N, Lapitskaya Y, Dima L, Manu P (August 2018). "Clozapine-Associated Pulmonary Embolism: A High-Mortality, Dose-Independent and Early-Onset Adverse Effect". American Journal of Therapeutics. 25 (4): e434–e438. doi:10.1097/MJT.0000000000000806. PMID 29985823. S2CID 51608744.
- Citrome L, McEvoy JP, Saklad SR (2016). "Guide to the Management of Clozapine-Related Tolerability and Safety Concerns". Clinical Schizophrenia & Related Psychoses. 10 (3): 163–177. doi:10.3371/1935-1232.10.3.163. PMID 27732102.
- Sriretnakumar V, Huang E, Müller DJ (2015). "Pharmacogenetics of clozapine treatment response and side-effects in schizophrenia: an update". Expert Opinion on Drug Metabolism & Toxicology. 11 (11): 1709–31. doi:10.1517/17425255.2015.1075003. PMID 26364648. S2CID 207492339.
- Adam RL, Sidi H, Midin M, et al. (2018). "The Role of Atypical Antipsychotics in Sexuality: Road to Recovery in Schizophrenia". Current Drug Targets. 19 (12): 1402–1411. doi:10.2174/1389450118666170502130126. PMID 28464773.
- Nakata Y, Kanahara N, Iyo M (December 2017). "Dopamine supersensitivity psychosis in schizophrenia: Concepts and implications in clinical practice". Journal of Psychopharmacology (Oxford, England). 31 (12): 1511–1518. doi:10.1177/0269881117728428. PMID 28925317. S2CID 1957881.
- Elkis H, Buckley PF (June 2016). "Treatment-Resistant Schizophrenia". The Psychiatric Clinics of North America. 39 (2): 239–65. doi:10.1016/j.psc.2016.01.006. PMID 27216902.
- Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH (13 January 2017). "Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review". BMC Psychiatry. 17 (1): 12. doi:10.1186/s12888-016-1177-y. PMC 5237235. PMID 28086761.
- Essali A, Al-Haj Haasan N, Li C, Rathbone J (January 2009). "Clozapine versus typical neuroleptic medication for schizophrenia". The Cochrane Database of Systematic Reviews. (1): CD000059. doi:10.1002/14651858.CD000059.pub2. PMC 7065592. PMID 19160174.CS1 maint: extra punctuation (link)
- Potkin SG, Kane JM, Correll CU, et al. (7 January 2020). "The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research". NPJ Schizophrenia. 6 (1): 1. doi:10.1038/s41537-019-0090-z. PMC 6946650. PMID 31911624.
- Sinclair DJ, Zhao S, Qi F, et al. (19 March 2019). "Electroconvulsive therapy for treatment-resistant schizophrenia". The Cochrane Database of Systematic Reviews. 3: CD011847. doi:10.1002/14651858.CD011847.pub2. PMC 6424225. PMID 30888709.
- Miyamoto S, Jarskog LF, Fleischhacker WW (November 2014). "New therapeutic approaches for treatment-resistant schizophrenia: a look to the future". Journal of Psychiatric Research. 58: 1–6. doi:10.1016/j.jpsychires.2014.07.001. PMID 25070124.
- Agarwal P, Sarris CE, Herschman Y, Agarwal N, Mammis A (December 2016). "Schizophrenia and neurosurgery: A dark past with hope of a brighter future". Journal of Clinical Neuroscience. 34: 53–58. doi:10.1016/j.jocn.2016.08.009. PMID 27634495. S2CID 6929780.
- Nucifora FC, Woznica E, Lee BJ, Cascella N, Sawa A (November 2019). "Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives". Neurobiology of Disease. 131: 104257. doi:10.1016/j.nbd.2018.08.016. PMC 6395548. PMID 30170114.
- Servonnet A, Samaha AN (February 2020). "Antipsychotic-evoked dopamine supersensitivity". Neuropharmacology. 163: 107630. doi:10.1016/j.neuropharm.2019.05.007. PMID 31077727. S2CID 147704473.
- Seeman MV (March 2020). "The Gut Microbiome and Treatment-Resistance in Schizophrenia". The Psychiatric Quarterly. 91 (1): 127–136. doi:10.1007/s11126-019-09695-4. PMID 31781943. S2CID 208329435.
- Pharoah F, Mari J, Rathbone J, Wong W (December 2010). Pharoah F (ed.). "Family intervention for schizophrenia". The Cochrane Database of Systematic Reviews. 12 (12): CD000088. doi:10.1002/14651858.CD000088.pub2. PMC 4204509. PMID 21154340.
- Medalia A, Choi J (September 2009). "Cognitive remediation in schizophrenia" (PDF). Neuropsychology Review. 19 (3): 353–64. doi:10.1007/s11065-009-9097-y. PMID 19444614. S2CID 11824026. Archived (PDF) from the original on 23 October 2016.
- Philipp R, Kriston L, Lanio J, et al. (2019). "Effectiveness of metacognitive interventions for mental disorders in adults-A systematic review and meta-analysis (METACOG)". Clin Psychology & Psychotherapy. 26 (2): 227–240. doi:10.1002/cpp.2345. PMID 30456821.
- Dixon LB, Dickerson F, Bellack AS, et al. (January 2010). "The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements". Schizophrenia Bulletin. 36 (1): 48–70. doi:10.1093/schbul/sbp115. PMC 2800143. PMID 19955389.
- Bond GR, Drake RE (June 2015). "The critical ingredients of assertive community treatment". World Psychiatry. 14 (2): 240–2. doi:10.1002/wps.20234. PMC 4471983. PMID 26043344.
- Smeerdijk M (2017). "[Using resource groups in assertive community treatment; literature review and recommendation]". Tijdschrift voor Psychiatrie. 59 (8): 466–473. PMID 28880347.
- Dieterich M (6 January 2017). "Intensive case management for severe mental illness". The Cochrane Database of Systematic Reviews. 1: CD007906. doi:10.1002/14651858.CD007906.pub3. PMC 6472672. PMID 28067944.
- Jauhar S, McKenna PJ, Radua J, et al. (January 2014). "Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias". The British Journal of Psychiatry (Review). 204 (1): 20–9. doi:10.1192/bjp.bp.112.116285. PMID 24385461.
- Jones C, Hacker D, Meaden A, et al. (November 2018). "Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia". The Cochrane Database of Systematic Reviews. 11: CD008712. doi:10.1002/14651858.CD008712.pub3. PMC 6516879. PMID 30480760.
- "Cognitive Behavioural Therapy for Psychosis: A Health Technology Assessment". Ontario Health Technology Assessment Series. 18 (5): 1–141. 2018. PMC 6235075. PMID 30443277.
- "CBT and treatment resistant schizophrenia". discover.dc.nihr.ac.uk. 20 November 2018. Retrieved 10 January 2020.
- Bastiampillai, Tarun; Allison, Stephen; Gupta, Arun (1 November 2016). "NICE guidelines for schizophrenia: can art therapy be justified?". The Lancet Psychiatry. 3 (11): 1016–1017. doi:10.1016/S2215-0366(16)30322-4. PMID 27794367.
- Ruddy R, Milnes D (October 2005). "Art therapy for schizophrenia or schizophrenia-like illnesses". The Cochrane Database of Systematic Reviews (4): CD003728. doi:10.1002/14651858.CD003728.pub2. PMID 16235338. Archived from the original on 27 October 2011.
- Ruddy RA, Dent-Brown K (January 2007). "Drama therapy for schizophrenia or schizophrenia-like illnesses". The Cochrane Database of Systematic Reviews (1): CD005378. doi:10.1002/14651858.CD005378.pub2. PMID 17253555. Archived from the original on 25 August 2011.
- Chien WT, Clifton AV, Zhao S, Lui S (4 April 2019). "Peer support for people with schizophrenia or other serious mental illness". The Cochrane Database of Systematic Reviews. 4: CD010880. doi:10.1002/14651858.CD010880.pub2. PMC 6448529. PMID 30946482.
- Girdler SJ, Confino JE, Woesner ME (15 February 2019). "Exercise as a Treatment for Schizophrenia: A Review". Psychopharmacology Bulletin. 49 (1): 56–69. PMC 6386427. PMID 30858639.
- Firth J (1 May 2017). "Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis". Schizophrenia Bulletin. 43 (3): 546–556. doi:10.1093/schbul/sbw115. PMC 5464163. PMID 27521348.
- Tréhout M, Dollfus S (December 2018). "[Physical activity in patients with schizophrenia: From neurobiology to clinical benefits]". L'Encephale. 44 (6): 538–547. doi:10.1016/j.encep.2018.05.005. PMID 29983176.
- "Quality statement 7: Promoting healthy eating, physical activity and smoking cessation | Psychosis and schizophrenia in adults | Quality standards | NICE". www.nice.org.uk.
- Firth J, Carney R, Stubbs B, et al. (17 October 2018). "Nutritional Deficiencies and Clinical Correlates in First-Episode Psychosis: A Systematic Review and Meta-analysis". Schizophrenia Bulletin. 44 (6): 1275–1292. doi:10.1093/schbul/sbx162. PMC 6192507. PMID 29206972.
- Martone, G (April 2018). "Enhancement of recovery from mental illness with l-methylfolate supplementation". Perspectives in Psychiatric Care. 54 (2): 331–334. doi:10.1111/ppc.12227. PMID 28597528.
- Firth J, Teasdale SB, Allott K, et al. (October 2019). "The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials". World Psychiatry : Official Journal of the World Psychiatric Association (WPA). 18 (3): 308–324. doi:10.1002/wps.20672. PMC 6732706. PMID 31496103.
- Erlangsen A, Eaton WW, Mortensen PB, Conwell Y (February 2012). "Schizophrenia--a predictor of suicide during the second half of life?". Schizophrenia Research. 134 (2–3): 111–7. doi:10.1016/j.schres.2011.09.032. PMC 3266451. PMID 22018943.
- Saha S, Chant D, McGrath J (October 2007). "A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?". Archives of General Psychiatry. 64 (10): 1123–31. doi:10.1001/archpsyc.64.10.1123. PMID 17909124.
- Goroll AH, Mulley AG (2011). Primary Care Medicine: Office Evaluation and Management of The Adult Patient: Sixth Edition. Lippincott Williams & Wilkins. p. Chapter 101. ISBN 978-1-4511-2159-9.
- Rizvi S, Gold J, Khan AM (5 August 2019). "Role of Naltrexone in Improving Compulsive Drinking in Psychogenic Polydipsia". Cureus. 11 (8): e5320. doi:10.7759/cureus.5320. PMC 6777931. PMID 31598428.
- Seeman MV (September 2019). "Schizophrenia Mortality: Barriers to Progress". The Psychiatric Quarterly. 90 (3): 553–563. doi:10.1007/s11126-019-09645-0. PMID 31147816. S2CID 170078453.
- Charlson FJ, Ferrari AJ, Santomauro DF, et al. (17 October 2018). "Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016". Schizophrenia Bulletin. 44 (6): 1195–1203. doi:10.1093/schbul/sby058. PMC 6192504. PMID 29762765.
- World Health Organization (2008). The global burden of disease : 2004 update ([Online-Ausg.] ed.). Geneva, Switzerland: World Health Organization. p. 35. ISBN 9789241563710.
- Warner R (July 2009). "Recovery from schizophrenia and the recovery model". Current Opinion in Psychiatry. 22 (4): 374–80. doi:10.1097/YCO.0b013e32832c920b. PMID 19417668. S2CID 26666000.
- Menezes NM, Arenovich T, Zipursky RB (October 2006). "A systematic review of longitudinal outcome studies of first-episode psychosis". Psychological Medicine. 36 (10): 1349–62. doi:10.1017/S0033291706007951. PMID 16756689. S2CID 23475454.
- Coyle JT (2017). "Schizophrenia: Basic and Clinical". Advances in Neurobiology. 15: 255–280. doi:10.1007/978-3-319-57193-5_9. ISBN 978-3-319-57191-1. PMID 28674984.
- Isaac M, Chand P, Murthy P (August 2007). "Schizophrenia outcome measures in the wider international community". The British Journal of Psychiatry. Supplement. 50: s71–7. doi:10.1192/bjp.191.50.s71. PMID 18019048.
- Cohen A, Patel V, Thara R, Gureje O (March 2008). "Questioning an axiom: better prognosis for schizophrenia in the developing world?". Schizophrenia Bulletin. 34 (2): 229–44. doi:10.1093/schbul/sbm105. PMC 2632419. PMID 17905787.
- Carlborg A, Winnerbäck K, Jönsson EG, Jokinen J, Nordström P (July 2010). "Suicide in schizophrenia". Expert Review of Neurotherapeutics. 10 (7): 1153–64. doi:10.1586/ern.10.82. PMID 20586695. S2CID 204385719.
- Tsoi DT, Porwal M, Webster AC (28 February 2013). "Interventions for smoking cessation and reduction in individuals with schizophrenia". The Cochrane Database of Systematic Reviews (2): CD007253. doi:10.1002/14651858.CD007253.pub3. PMC 6486303. PMID 23450574.
- de Leon J, Diaz FJ (July 2005). "A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors". Schizophrenia Research. 76 (2–3): 135–57. doi:10.1016/j.schres.2005.02.010. PMID 15949648. S2CID 32975940.
- Keltner NL, Grant JS (November 2006). "Smoke, smoke, smoke that cigarette". Perspectives in Psychiatric Care. 42 (4): 256–61. doi:10.1111/j.1744-6163.2006.00085.x. PMID 17107571.
- Kumari V, Postma P (January 2005). "Nicotine use in schizophrenia: the self medication hypotheses". Neuroscience and Biobehavioral Reviews. 29 (6): 1021–34. doi:10.1016/j.neubiorev.2005.02.006. PMID 15964073. S2CID 15581894.
- Cascio MT, Cella M, Preti A, Meneghelli A, Cocchi A (May 2012). "Gender and duration of untreated psychosis: a systematic review and meta-analysis". Early Intervention in Psychiatry (Review). 6 (2): 115–27. doi:10.1111/j.1751-7893.2012.00351.x. PMID 22380467.
- Jablensky A, Sartorius N, Ernberg G, et al. (1992). "Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study". Psychological Medicine. Monograph Supplement. 20: 1–97. doi:10.1017/S0264180100000904. PMID 1565705. S2CID 44841074.
- Kirkbride JB, Fearon P, Morgan C, et al. (March 2006). "Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study". Archives of General Psychiatry. 63 (3): 250–8. doi:10.1001/archpsyc.63.3.250. PMID 16520429.
- Kirkbride JB, Fearon P, Morgan C, et al. (June 2007). "Neighbourhood variation in the incidence of psychotic disorders in Southeast London". Social Psychiatry and Psychiatric Epidemiology. 42 (6): 438–45. doi:10.1007/s00127-007-0193-0. PMID 17473901. S2CID 19655724.
- Ayuso-Mateos JL. "Global burden of schizophrenia in the year 2000" (PDF). World Health Organization. Archived (PDF) from the original on 4 March 2016. Retrieved 27 February 2013.
- "Schizophrenia". Archived from the original on 4 October 2016. Retrieved 29 December 2015.
- Charlson F, van Ommeren M, Flaxman A, et al. (20 July 2019). "New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis". Lancet. 394 (10194): 240–248. doi:10.1016/S0140-6736(19)30934-1. PMC 6657025. PMID 31200992.
- Yuhas D. "Throughout History, Defining Schizophrenia Has Remained a Challenge". Scientific American Mind (March/April 2013). Archived from the original on 5 November 2013. Retrieved 3 March 2013.
- Heinrichs RW (2003). "Historical origins of schizophrenia: two early madmen and their illness". Journal of the History of the Behavioral Sciences. 39 (4): 349–63. doi:10.1002/jhbs.10152. PMID 14601041.
- Noll R (2012). "Whole body madness". Psychiatric Times. 29 (12): 13–14. Archived from the original on 11 January 2013.
- Kuhn R, Cahn CH (September 2004). "Eugen Bleuler's concepts of psychopathology". History of Psychiatry. 15 (59 Pt 3): 361–6. doi:10.1177/0957154X04044603. PMID 15386868.
- "Schizophrenia | Definition of Schizophrenia by Lexico". Lexico Dictionaries | English.
- McNally K (2016). "The Split Personality". A Critical History of Schizophrenia. Palgrave Macmillan UK. pp. 21–38. doi:10.1057/9781137456816_3. ISBN 978-1-349-55226-9.
- Sato M (February 2006). "Renaming schizophrenia: a Japanese perspective". World Psychiatry. 5 (1): 53–5. PMC 1472254. PMID 16757998.
- Park SC, Park YC (13 January 2020). "Korea in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition". Journal of Korean Medical Science. 35 (2): e6. doi:10.3346/jkms.2020.35.e6. PMC 6955430. PMID 31920014.
- Schneider K (1959). Clinical Psychopathology (5 ed.). New York: Grune & Stratton.
- Moore D (25 April 2008). Textbook of Clinical Neuropsychiatry, Second Edition. CRC Press. ISBN 9781444109740. Retrieved 9 February 2020.
- Picardi A (2019). "The Two Faces of First-Rank Symptoms". Psychopathology. 52 (4): 221–231. doi:10.1159/000503152. PMID 31610542. S2CID 204702486.
- Mashour GA, Walker EE, Martuza RL (June 2005). "Psychosurgery: past, present, and future". Brain Research. Brain Research Reviews. 48 (3): 409–19. doi:10.1016/j.brainresrev.2004.09.002. PMID 15914249. S2CID 10303872.
- Jobes, DA; Chalker, SA (26 September 2019). "One Size Does Not Fit All: A Comprehensive Clinical Approach to Reducing Suicidal Ideation, Attempts, and Deaths". International Journal of Environmental Research and Public Health. 16 (19): 3606. doi:10.3390/ijerph16193606. PMC 6801408. PMID 31561488.
- Jones K (March 2000). "Insulin coma therapy in schizophrenia". Journal of the Royal Society of Medicine. 93 (3): 147–9. doi:10.1177/014107680009300313. PMC 1297956. PMID 10741319.
- Ali SA, Mathur N, Malhotra AK, Braga RJ (April 2019). "Electroconvulsive Therapy and Schizophrenia: A Systematic Review". Molecular Neuropsychiatry. 5 (2): 75–83. doi:10.1159/000497376. PMC 6528094. PMID 31192220.
- Turner T (January 2007). "Chlorpromazine: unlocking psychosis". BMJ. 334 Suppl 1 (suppl): s7. doi:10.1136/bmj.39034.609074.94. PMID 17204765.
- Aringhieri S (December 2018). "Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences". Pharmacology & Therapeutics. 192: 20–41. doi:10.1016/j.pharmthera.2018.06.012. PMID 29953902. S2CID 49602956.
- Wilson M (March 1993). "DSM-III and the transformation of American psychiatry: a history". The American Journal of Psychiatry. 150 (3): 399–410. doi:10.1176/ajp.150.3.399. PMID 8434655.
- Fischer BA (December 2012). "A review of American psychiatry through its diagnoses: the history and development of the Diagnostic and Statistical Manual of Mental Disorders". The Journal of Nervous and Mental Disease. 200 (12): 1022–30. doi:10.1097/NMD.0b013e318275cf19. PMID 23197117. S2CID 41939669.
- "Schizophrenia". University of Michigan Department of Psychiatry. Archived from the original on 3 April 2013. Retrieved 24 June 2013.
- Reed GM, First MB, Kogan CS, et al. (February 2019). "Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders". World Psychiatry. 18 (1): 3–19. doi:10.1002/wps.20611. PMC 6313247. PMID 30600616.
- "Updates to DSM-5 Criteria & Text". www.psychiatry.org. Retrieved 21 February 2019.
- Tandon, Rajiv (2014). "Schizophrenia and Other Psychotic Disorders in Diagnostic and Statistical Manual of Mental Disorders (DSM)-5: Clinical Implications of Revisions from DSM-IV". Indian Journal of Psychological Medicine. 36 (3): 223–225. doi:10.4103/0253-7176.135365. ISSN 0253-7176. PMC 4100404. PMID 25035542.
- Yamaguchi S, Mizuno M, Ojio Y, et al. (June 2017). "Associations between renaming schizophrenia and stigma-related outcomes: A systematic review". Psychiatry and Clinical Neurosciences. 71 (6): 347–362. doi:10.1111/pcn.12510. PMID 28177184.
- van Os J (February 2016). ""Schizophrenia" does not exist". BMJ. 352: i375. doi:10.1136/bmj.i375. PMID 26837945.
- Tan N, van Os J (2014). "[The schizophrenia spectrum and other psychotic disorders in the DSM-5]". Tijdschrift voor Psychiatrie. 56 (3): 167–72. PMID 24643825.
- Wu EQ, Birnbaum HG, Shi L, et al. (September 2005). "The economic burden of schizophrenia in the United States in 2002". The Journal of Clinical Psychiatry. 66 (9): 1122–9. doi:10.4088/jcp.v66n0906. PMID 16187769.
- Dyga K, Stupak R (28 February 2018). "Ways of understanding of religious delusions associated with a change of identity on the example of identification with Jesus Christ". Psychiatria Polska. 52 (1): 69–80. doi:10.12740/PP/64378. PMID 29704415.
- Dein S, Littlewood R (July 2011). "Religion and psychosis: a common evolutionary trajectory?". Transcultural Psychiatry. 48 (3): 318–35. doi:10.1177/1363461511402723. PMID 21742955.
- Maniglio R (March 2009). "Severe mental illness and criminal victimization: a systematic review". Acta Psychiatrica Scandinavica. 119 (3): 180–91. doi:10.1111/j.1600-0447.2008.01300.x. PMID 19016668.
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M (August 2009). "Schizophrenia and violence: systematic review and meta-analysis". PLOS Medicine. 6 (8): e1000120. doi:10.1371/journal.pmed.1000120. PMC 2718581. PMID 19668362.
- Large M, Smith G, Nielssen O (July 2009). "The relationship between the rate of homicide by those with schizophrenia and the overall homicide rate: a systematic review and meta-analysis". Schizophrenia Research. 112 (1–3): 123–9. doi:10.1016/j.schres.2009.04.004. PMID 19457644. S2CID 23843470.
- Bo S, Abu-Akel A, Kongerslev M, Haahr UH, Simonsen E (July 2011). "Risk factors for violence among patients with schizophrenia". Clinical Psychology Review. 31 (5): 711–26. doi:10.1016/j.cpr.2011.03.002. PMID 21497585.
- Valença AM, de Moraes TM (October 2006). "[Relationship between homicide and mental disorders]". Revista Brasileira de Psiquiatria. 28 Suppl 2: S62–8. doi:10.1590/s1516-44462006000600003. PMID 17143446.
- Pescosolido BA, Monahan J, Link BG, Stueve A, Kikuzawa S (September 1999). "The public's view of the competence, dangerousness, and need for legal coercion of persons with mental health problems". American Journal of Public Health. 89 (9): 1339–45. doi:10.2105/AJPH.89.9.1339. PMC 1508769. PMID 10474550.
- Phelan JC, Link BG, Stueve A, Pescosolido BA (June 2000). "Public Conceptions of Mental Illness in 1950 and 1996: What Is Mental Illness and Is It to be Feared?". Journal of Health and Social Behavior. 41 (2): 188–207. doi:10.2307/2676305. JSTOR 2676305. S2CID 31634899.
- Stetka, Bret (24 March 2015). "Why Don't Animals Get Schizophrenia (and How Come We Do)?". Scientific American.
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD (September 2013). "Nonhuman primate model of schizophrenia using a noninvasive EEG method". Proceedings of the National Academy of Sciences of the United States of America. 110 (38): 15425–30. Bibcode:2013PNAS..11015425G. doi:10.1073/pnas.1312264110. PMC 3780912. PMID 23959894.
- Fusar-Poli, P, Rutigliano, G, Stahl, D, Davies, C, Bonoldi, I, Reilly, T, McGuire, P (2017). "Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis". JAMA psychiatry. 74 (5): 493–500. doi:10.1001/jamapsychiatry.2017.0284.
- Raket LL, Jaskolowski J, Kinon BJ, Brasen JC, Jönsson L, Wehnert A, Fusar-Poli P (2020). "Dynamic ElecTronic hEalth reCord deTection (DETECT) of individuals at risk of a first episode of psychosis: a case-control development and validation study". The Lancet Digital Health. 2 (5): e229–e239. doi:10.1016/S2589-7500(20)30024-8.
- Chue P, Lalonde JK (2014). "Addressing the unmet needs of patients with persistent negative symptoms of schizophrenia: emerging pharmacological treatment options". Neuropsychiatric Disease and Treatment. 10: 777–89. doi:10.2147/ndt.s43404. PMC 4020880. PMID 24855363.
- Keller WR, Kum LM, Wehring HJ, et al. (April 2013). "A review of anti-inflammatory agents for symptoms of schizophrenia". Journal of Psychopharmacology. 27 (4): 337–42. doi:10.1177/0269881112467089. PMC 3641824. PMID 23151612.
- Xiang YQ, et al. (January 2017). "Adjunctive minocycline for schizophrenia: A meta-analysis of randomized controlled trials". European Neuropsychopharmacology. 27 (1): 8–18. doi:10.1016/j.euroneuro.2016.11.012. PMID 27919523. S2CID 4527988.
- Solmi M (October 2017). "Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia". CNS Spectrums. 22 (5): 415–426. doi:10.1017/S1092852916000638. PMID 28181901.
- Chamberlain IJ, Sampson S (March 2013). Chamberlain IJ (ed.). "Nidotherapy for people with schizophrenia". The Cochrane Database of Systematic Reviews. 3 (3): CD009929. doi:10.1002/14651858.CD009929.pub2. PMC 6492500. PMID 23543583.
- Nieuwdorp W, Koops S, Somers M, Sommer IE (May 2015). "Transcranial magnetic stimulation, transcranial direct current stimulation and electroconvulsive therapy for medication-resistant psychosis of schizophrenia". Current Opinion in Psychiatry. 28 (3): 222–8. doi:10.1097/YCO.0000000000000156. PMID 25768083. S2CID 206141551.
- Nathou C, Etard O, Dollfus S (2019). "Auditory verbal hallucinations in schizophrenia: current perspectives in brain stimulation treatments". Neuropsychiatric Disease and Treatment. 15: 2105–2117. doi:10.2147/NDT.S168801. PMC 6662171. PMID 31413576.
- Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A (20 August 2015). "Transcranial magnetic stimulation (TMS) for schizophrenia". The Cochrane Database of Systematic Reviews. 2015 (8): CD006081. doi:10.1002/14651858.CD006081.pub2. hdl:1893/22520. PMID 26289586.
- Goldsmith DR, Crooks CL, Walker EF, Cotes RO (April 2018). "An Update on Promising Biomarkers in Schizophrenia". Focus (American Psychiatric Publishing). 16 (2): 153–163. doi:10.1176/appi.focus.20170046. PMC 6526854. PMID 31975910.
External links
| Classification | |
|---|---|
| External resources |