Ribonuclease H
Ribonuclease H (abbreviated RNase H or RNH) is a family of non-sequence-specific endonuclease enzymes that catalyze the cleavage of RNA in an RNA/DNA substrate via a hydrolytic mechanism. Members of the RNase H family can be found in nearly all organisms, from bacteria to archaea to eukaryotes.
| ribonuclease H | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 3.1.26.4 | ||||||||
| CAS number | 9050-76-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| retroviral ribonuclease H | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 3.1.26.13 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
The family is divided into evolutionarily related groups with slightly different substrate preferences, broadly designated ribonuclease H1 and H2.[2] The human genome encodes both H1 and H2. Human ribonuclease H2 is a heterotrimeric complex composed of three subunits, mutations in any of which are among the genetic causes of a rare disease known as Aicardi–Goutières syndrome.[3] A third type, closely related to H2, is found only in a few prokaryotes,[4] whereas H1 and H2 occur in all domains of life.[4] Additionally, RNase H1-like retroviral ribonuclease H domains occur in multidomain reverse transcriptase proteins, which are encoded by retroviruses such as HIV and are required for viral replication.[5][6]
In eukaryotes, ribonuclease H1 is involved in DNA replication of the mitochondrial genome. Both H1 and H2 are involved in genome maintenance tasks such as processing of R-loop structures.[2][7]
Classification and nomenclature
Ribonuclease H is a family of endonuclease enzymes with a shared substrate specificity for the RNA strand of RNA-DNA duplexes. By definition, RNases H cleave RNA backbone phosphodiester bonds to leave a 3' hydroxyl and a 5' phosphate group.[7] RNases H have been proposed as members of an evolutionarily related superfamily encompassing other nucleases and nucleic acid processing enzymes such as retroviral integrases, DNA transposases, Holliday junction resolvases, Piwi and Argonaute proteins, various exonucleases, and the spliceosomal protein Prp8.[8][9]
RNases H can be broadly divided into two subtypes, H1 and H2, which for historical reasons are given Arabic numeral designations in eukaryotes and Roman numeral designations in prokaryotes. Thus the Escherichia coli RNase HI is a homolog of the Homo sapiens RNase H1.[2][7] In E. coli and many other prokaryotes, the rnhA gene encodes HI and the rnhB gene encodes HII. A third related class, called HIII, occurs in a few bacteria and archaea; it is closely related to prokaryotic HII enzymes.[4]
Structure
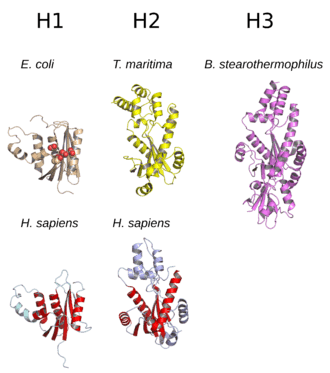
The structure of RNase H commonly consists of a 5-stranded β-sheet surrounded by a distribution of α-helices.[10] All RNases H have an active site centered on a conserved sequence motif composed of aspartate and glutamate residues, often referred to as the DEDD motif. These residues interact with catalytically required magnesium ions.[7][5]
RNases H2 are larger than H1 and usually have additional helices. The domain organization of the enzymes varies; some prokaryotic and most eukaryotic members of the H1 group have an additional small domain at the N-terminus known as the "hybrid binding domain", which facilitates binding to RNA:DNA hybrid duplexes and sometimes confers increased processivity.[2][7][11] While all members of the H1 group and the prokaryotic members of the H2 group function as monomers, eukaryotic H2 enzymes are obligate heterotrimers.[2][7] Prokaryotic HIII enzymes are members of the broader H2 group and share most structural features with H2, with the addition of an N-terminal TATA box binding domain.[7] Retroviral RNase H domains occurring in multidomain reverse transcriptase proteins have structures closely resembling the H1 group.[5]
RNases H1 have been extensively studied to explore the relationships between structure and enzymatic activity. They are also used, especially the E. coli homolog, as model systems to study protein folding.[12][13][14] Within the H1 group, a relationship has been identified between higher substrate-binding affinity and the presence of structural elements consisting of a helix and flexible loop providing a larger and more basic substrate-binding surface. The C-helix has a scattered taxonomic distribution; it is present in the E. coli and human RNase H1 homologs and absent in the HIV RNase H domain, but examples of retroviral domains with C-helices do exist.[15][16]
Function
Ribonuclease H enzymes cleave the phosphodiester bonds of RNA in a double-stranded RNA:DNA hybrid, leaving a 3' hydroxyl and a 5' phosphate group on either end of the cut site with a two-metal-ion catalysis mechanism, in which two divalent cations, such as Mg2+ and Mn2+, directly participate in the catalytic function.[17] Depend on the differences in their amino acid sequences, these RNases H are classified into type 1 and type 2 RNases H.[18][19] Type 1 RNases H have prokaryotic and eukaryotic RNases H1 and retroviral RNase H. Type 2 RNases H have prokaryotic and eukaryotic RNases H2 and bacterial RNase H3. These RNases H exist in a monomeric form , except for eukaryotic RNases H2, which exist in a heterotrimeric form. [20][21]RNase H1 and H2 have distinct substrate preferences and distinct but overlapping functions in the cell. In prokaryotes and lower eukaryotes, neither enzyme is essential, whereas both are believed to be essential in higher eukaryotes.[2] The combined activity of both H1 and H2 enzymes is associated with maintenance of genome stability due to the enzymes' degradation of the RNA component of R-loops.[22][23]
Ribonuclease H1
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | RNase H | ||||||||
| Pfam | PF00075 | ||||||||
| Pfam clan | CL0219 | ||||||||
| InterPro | IPR002156 | ||||||||
| PROSITE | PS50879 | ||||||||
| |||||||||
Ribonuclease H1 enzymes require at least four ribonucleotide-containing base pairs in a substrate and cannot remove a single ribonucleotide from a strand that is otherwise composed of deoxyribonucleotides. For this reason, it is considered unlikely that RNase H1 enzymes are involved in the processing of RNA primers from Okazaki fragments during DNA replication.[2] RNase H1 is not essential in unicellular organisms where it has been investigated; in E. coli, RNase H1 knockouts confer a temperature-sensitive phenotype,[7] and in S. cerevisiae, they produce defects in stress response.[24]
In many eukaryotes, including mammals, RNase H1 genes include a mitochondrial targeting sequence, leading to expression of isoforms with and without the MTS present. As a result, RNase H1 is localized to both mitochondria and the nucleus. In knockout mouse models, RNase H1-null mutants are lethal during embryogenesis due to defects in replicating mitochondrial DNA.[2][25][26] The defects in mitochondrial DNA replication induced by loss of RNase H1 are likely due to defects in R-loop processing.[23]
Ribonuclease H2
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | RNase HII | ||||||||
| Pfam | PF01351 | ||||||||
| Pfam clan | CL0219 | ||||||||
| InterPro | IPR024567 | ||||||||
| |||||||||
In prokaryotes, RNase H2 is enzymatically active as a monomeric protein. In eukaryotes, it is an obligate heterotrimer composed of a catalytic subunit A and structural subunits B and C. While the A subunit is closely homologous to the prokaryotic RNase H2, the B and C subunits have no apparent homologs in prokaryotes and are poorly conserved at the sequence level even among eukaryotes.[27][28] The B subunit mediates protein-protein interactions between the H2 complex and PCNA, which localizes H2 to replication foci.[29]
Both prokaryotic and eukaryotic H2 enzymes can cleave single ribonucleotides in a strand.[2] however, they have slightly different cleavage patterns and substrate preferences: prokaryotic enzymes have lower processivity and hydrolyze successive ribonucleotides more efficiently than ribonucleotides with a 5' deoxyribonucleotide, while eukaryotic enzymes are more processive and hydrolyze both types of substrate with similar efficiency.[2][30] The substrate specificity of RNase H2 gives it a role in ribonucleotide excision repair, removing misincorporated ribonucleotides from DNA, in addition to R-loop processing.[31][32][29] Although both H1 and H2 are present in the mammalian cell nucleus, H2 is the dominant source of RNase H activity there and is important for maintaining genome stability.[29]
Some prokaryotes possess an additional H2-type gene designated RNase HIII in the Roman-numeral nomenclature used for the prokaryotic genes. HIII proteins are more closely related to the H2 group by sequence identity and structural similarity, but have substrate preferences that more closely resemble H1.[7][33] Unlike HI and HII, which are both widely distributed among prokaryotes, HIII is found in only a few organisms with a scattered taxonomic distribution; it is somewhat more common in archaea and is rarely or never found in the same prokaryotic genome as HI.[34]
Mechanism
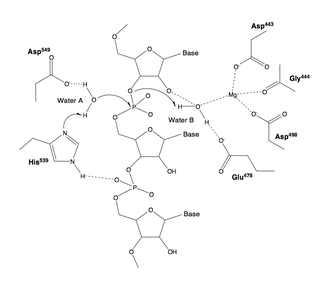
The active site of nearly all RNases H contains four negatively charged amino acid residues, known as the DEDD motif; often histidine is also present.[2][7]
The charged residues bind either one or two metal ions that are required for catalysis; under physiological conditions these are magnesium ions, but manganese also usually supports enzymatic activity,[2][7] while calcium may inhibit it.[11][35] Although two-metal-ion catalytic mechanisms are very common in enzymes involved in phosphate biochemistry, it has been a subject of debate in the literature whether one or two ions are used in RNase H catalysis. In either proposed mechanism, at least one water molecule participates in the reaction.[36][37]
Most experimental evidence for the mechanism of RNase H catalysis comes from measurements performed on members of the H1 group, usually the E. coli homolog. According to measurements of this protein, one of the aspartate residues has an elevated pKa, while another has an abnormally low pKa.[38] It is unclear whether any of the active-site residues participates in the reaction as a general base.[7] In addition, it is possible that one of the substrate's oxygen atoms participates directly in the reaction as a base.[39]
In human biology
The human genome contains four genes encoding RNase H:
- RNASEH1, an example of the H1 (monomeric) subtype
- RNASEH2A, the catalytic subunit of the trimeric H2 complex
- RNASEH2B, a structural subunit of the trimeric H2 complex
- RNASEH2C, a structural subunit of the trimeric H2 complex
In addition, genetic material of retroviral origin appears frequently in the genome, reflecting integration of the genomes of human endogenous retroviruses. Such integration events result in the presence of genes encoding retroviral reverse transcriptase, which includes an RNase H domain. An example is ERVK6.[40] Long terminal repeat (LTR) and non-long terminal repeat (non-LTR) retrotransposons are also common in the genome and often include their own RNase H domains, with a complex evolutionary history.[41][42][43]
Role in disease
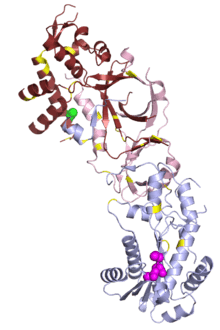
In small studies, mutations in human RNase H1 have been associated with chronic progressive external ophthalmoplegia, a common feature of mitochondrial disease.[26]
Mutations in any of the three RNase H2 subunits are well-established as causes of a rare genetic disorder known as Aicardi–Goutières syndrome (AGS),[3] which manifests as neurological and dermatological symptoms at an early age.[45] The symptoms of AGS closely resemble those of congenital viral infection and are associated with inappropriate upregulation of type I interferon. AGS can also be caused by mutations in other genes: TREX1, SAMHD1, ADAR, and MDA5/IFIH1, all of which are involved in nucleic acid processing.[46] Characterization of mutational distribution in an AGS patient population found 5% of all AGS mutations in RNASEH2A, 36% in 2B, and 12% in 2C.[47] Mutations in 2B have been associated with somewhat milder neurological impairment[48] and with an absence of interferon-induced gene upregulation that can be detected in patients with other AGS-associated genotypes.[46]
In viruses
Two groups of viruses use reverse transcription as part of their life cycles: retroviruses, which encode their genomes in single-stranded RNA and replicate through a double-stranded DNA intermediate; and dsDNA-RT viruses, which replicate their double-stranded DNA genomes through an RNA "pregenome" intermediate. Pathogenic examples include human immunodeficiency virus and hepatitis B virus, respectively. Both encode large multifunctional reverse transcriptase (RT) proteins containing RNase H domains.[50][51]
Retroviral RT proteins from HIV-1 and murine leukemia virus are the best-studied members of the family.[52][53] Retroviral RT is responsible for converting the virus' single-stranded RNA genome into double-stranded DNA. This process requires three steps: first, RNA-dependent DNA polymerase activity produces minus-strand DNA from the plus-strand RNA template, generating an RNA:DNA hybrid intermediate; second, the RNA strand is destroyed; and third, DNA-dependent DNA polymerase activity synthesizes plus-strand DNA, generating double-stranded DNA as the final product. The second step of this process is carried out by an RNase H domain located at the C-terminus of the RT protein.[5][6][54][55]
RNase H performs three types of cleaving actions: non-specific degradation of the plus-strand RNA genome, specific removal of the minus-strand tRNA primer, and removal of the plus-strand purine-rich polypurine tract (PPT) primer.[56] RNase H plays a role in the priming of the plus-strand, but not in the conventional method of synthesizing a new primer sequence. Rather RNase H creates a "primer" from the PPT that is resistant to RNase H cleavage. By removing all bases but the PPT, the PPT is used as a marker for the end of the U3 region of its long terminal repeat.[55]
Because RNase H activity is required for viral proliferation, this domain has been considered a drug target for the development of antiretroviral drugs used in the treatment of HIV/AIDS and other conditions caused by retroviruses. Inhibitors of retroviral RNase H of several different chemotypes have been identified, many of which have a mechanism of action based on chelation of the active-site cations.[57] Reverse-transcriptase inhibitors that specifically inhibit the polymerase function of RT are in widespread clinical use, but not inhibitors of the RNase H function; it is the only enzymatic function encoded by HIV that is not yet targeted by drugs in clinical use.[54][58]
Evolution
RNases H are widely distributed and occur in all domains of life. The family belongs to a larger superfamily of nuclease enzymes[8][9] and is considered to be evolutionarily ancient.[59] In prokaryotic genomes, multiple RNase H genes are often present, but there is little correlation between occurrence of HI, HII, and HIII genes and overall phylogenetic relationships, suggesting that horizontal gene transfer may have played a role in establishing the distribution of these enzymes. RNase HI and HIII rarely or never appear in the same prokaryotic genome. When an organism's genome contains more than one RNase H gene, they sometimes have significant differences in activity level. These observations have been suggested to reflect an evolutionary pattern that minimizes functional redundancy among RNase H genes.[7][34] RNase HIII, which is unique to prokaryotes, has a scattered taxonomic distribution and is found in both bacteria and archaea;[34] it is believed to have diverged from HII fairly early.[60]
The evolutionary trajectory of RNase H2 in eukaryotes, especially the mechanism by which eukaryotic homologs became obligate heterotrimers, is unclear; the B and C subunits have no apparent homologs in prokaryotes.[2][28]
Applications
Because RNase H specifically degrades only the RNA in double-stranded RNA:DNA hybrids, it is commonly used as a laboratory reagent in molecular biology. Purified preparations of E. coli RNase HI and HII are commercially available. RNase HI is often used to destroy the RNA template after first-strand complementary DNA (cDNA) synthesis by reverse transcription. It can also be used to cleave specific RNA sequences in the presence of short complementary segments of DNA.[61] Highly sensitive techniques such as surface plasmon resonance can be used for detection.[62][63] RNase HII can be used to degrade the RNA primer component of an Okazaki fragment or to introduce single-stranded nicks at positions containing a ribonucleotide.[61] A variant of hot start PCR, known as RNase H-dependent PCR or rhPCR, has been described using a thermostable RNase HII from the hyperthermophilic archaeon Pyrococcus abyssi.[64] Of note, the ribonuclease inhibitor protein commonly used as a reagent is not effective at inhibiting the activity of either HI or HII.[61]
History
Ribonucleases H were first discovered in the laboratory of Peter Hausen when researchers found RNA:DNA hybrid endonuclease activity in calf thymus in 1969 and gave it the name "ribonuclease H" to designate its hybrid specificity.[27][65][66] RNase H activity was subsequently discovered in E. coli[67] and in a sample of oncoviruses with RNA genomes during early studies of viral reverse transcription.[68][69] It later became clear that calf thymus extract contained more than one protein with RNase H activity[70] and that E. coli contained two RNase H genes.[71][72] Originally, the enzyme now known as RNase H2 in eukaryotes was designated H1 and vice versa, but the names of the eukaryotic enzymes were switched to match those in E. coli to facilitate comparative analysis, yielding the modern nomenclature in which the prokaryotic enzymes are designated with Roman numerals and the eukaryotic enzymes with Arabic numerals.[2][27][73][74] The prokaryotic RNase HIII, reported in 1999, was the last RNase H subtype to be identified.[73]
Characterizing eukaryotic RNase H2 was historically a challenge, in part due to its low abundance.[2] Careful efforts at purification of the enzyme suggested that, unlike the E. coli RNase H2, the eukaryotic enzyme had multiple subunits.[75] The S. cerevisiae homolog of the E. coli protein (that is, the H2A subunit) was easily identifiable by bioinformatics when the yeast genome was sequenced,[76] but the corresponding protein was found not to have enzymatic activity in isolation.[2][24] Eventually, the yeast B and C subunits were isolated by co-purification and found to be required for enzymatic activity.[77] However, the yeast B and C subunits have very low sequence identity to their homologs in other organisms, and the corresponding human proteins were conclusively identified only after mutations in all three were found to cause Aicardi–Goutières syndrome.[2][3]
References
- PDB: 1JL1; Goedken ER, Marqusee S (December 2001). "Native-state energetics of a thermostabilized variant of ribonuclease HI". Journal of Molecular Biology. 314 (4): 863–71. doi:10.1006/jmbi.2001.5184. PMID 11734003.
- Cerritelli SM, Crouch RJ (March 2009). "Ribonuclease H: the enzymes in eukaryotes". The FEBS Journal. 276 (6): 1494–505. doi:10.1111/j.1742-4658.2009.06908.x. PMC 2746905. PMID 19228196.
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, et al. (August 2006). "Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection". Nature Genetics. 38 (8): 910–6. doi:10.1038/ng1842. PMID 16845400.
- Figiel M, Nowotny M (August 2014). "Crystal structure of RNase H3-substrate complex reveals parallel evolution of RNA/DNA hybrid recognition". Nucleic Acids Research. 42 (14): 9285–94. doi:10.1093/nar/gku615. PMC 4132731. PMID 25016521.
- Davies JF, Hostomska Z, Hostomsky Z, Jordan SR, Matthews DA (April 1991). "Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase". Science. 252 (5002): 88–95. Bibcode:1991Sci...252...88D. doi:10.1126/science.1707186. PMID 1707186.
- Hansen J, Schulze T, Mellert W, Moelling K (January 1988). "Identification and characterization of HIV-specific RNase H by monoclonal antibody". The EMBO Journal. 7 (1): 239–43. doi:10.1002/j.1460-2075.1988.tb02805.x. PMC 454263. PMID 2452083.
- Tadokoro T, Kanaya S (March 2009). "Ribonuclease H: molecular diversities, substrate binding domains, and catalytic mechanism of the prokaryotic enzymes". The FEBS Journal. 276 (6): 1482–93. doi:10.1111/j.1742-4658.2009.06907.x. PMID 19228197.
- Majorek KA, Dunin-Horkawicz S, Steczkiewicz K, Muszewska A, Nowotny M, Ginalski K, Bujnicki JM (April 2014). "The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification". Nucleic Acids Research. 42 (7): 4160–79. doi:10.1093/nar/gkt1414. PMC 3985635. PMID 24464998.
- Rice P, Craigie R, Davies DR (February 1996). "Retroviral integrases and their cousins". Current Opinion in Structural Biology. 6 (1): 76–83. doi:10.1016/s0959-440x(96)80098-4. PMID 8696976.
- Schmitt TJ, Clark JE, Knotts TA (December 2009). "Thermal and mechanical multistate folding of ribonuclease H". The Journal of Chemical Physics. 131 (23): 235101. Bibcode:2009JChPh.131w5101S. doi:10.1063/1.3270167. PMID 20025349.
- Nowotny M, Cerritelli SM, Ghirlando R, Gaidamakov SA, Crouch RJ, Yang W (April 2008). "Specific recognition of RNA/DNA hybrid and enhancement of human RNase H1 activity by HBD". The EMBO Journal. 27 (7): 1172–81. doi:10.1038/emboj.2008.44. PMC 2323259. PMID 18337749.
- Cecconi C, Shank EA, Bustamante C, Marqusee S (September 2005). "Direct observation of the three-state folding of a single protein molecule". Science. 309 (5743): 2057–60. Bibcode:2005Sci...309.2057C. doi:10.1126/science.1116702. PMID 16179479.
- Hollien J, Marqusee S (March 1999). "A thermodynamic comparison of mesophilic and thermophilic ribonucleases H". Biochemistry. 38 (12): 3831–6. doi:10.1021/bi982684h. PMID 10090773.
- Raschke TM, Marqusee S (April 1997). "The kinetic folding intermediate of ribonuclease H resembles the acid molten globule and partially unfolded molecules detected under native conditions". Nature Structural Biology. 4 (4): 298–304. doi:10.1038/nsb0497-298. PMID 9095198.
- Schultz SJ, Champoux JJ (June 2008). "RNase H activity: structure, specificity, and function in reverse transcription". Virus Research. 134 (1–2): 86–103. doi:10.1016/j.virusres.2007.12.007. PMC 2464458. PMID 18261820.
- Champoux JJ, Schultz SJ (March 2009). "Ribonuclease H: properties, substrate specificity and roles in retroviral reverse transcription". The FEBS Journal. 276 (6): 1506–16. doi:10.1111/j.1742-4658.2009.06909.x. PMC 2742777. PMID 19228195.
- Yang W, Lee JY, Nowotny M (April 2006). "Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity". Molecular Cell. 22 (1): 5–13. doi:10.1016/j.molcel.2006.03.013. PMID 16600865.
- Tadokoro T, Kanaya S (March 2009). "Ribonuclease H: molecular diversities, substrate binding domains, and catalytic mechanism of the prokaryotic enzymes". The FEBS Journal. 276 (6): 1482–93. doi:10.1111/j.1742-4658.2009.06907.x. PMID 19228197.
- Ohtani N, Haruki M, Morikawa M, Kanaya S (January 1999). "Molecular diversities of RNases H". Journal of Bioscience and Bioengineering. 88 (1): 12–9. doi:10.1016/s1389-1723(99)80168-6. PMID 16232566.
- Bubeck D, Reijns MA, Graham SC, Astell KR, Jones EY, Jackson AP (May 2011). "PCNA directs type 2 RNase H activity on DNA replication and repair substrates". Nucleic Acids Research. 39 (9): 3652–66. doi:10.1093/nar/gkq980. PMC 3089482. PMID 21245041.
- Figiel M, Chon H, Cerritelli SM, Cybulska M, Crouch RJ, Nowotny M (March 2011). "The structural and biochemical characterization of human RNase H2 complex reveals the molecular basis for substrate recognition and Aicardi-Goutières syndrome defects". The Journal of Biological Chemistry. 286 (12): 10540–50. doi:10.1074/jbc.M110.181974. PMC 3060507. PMID 21177858.
- Amon JD, Koshland D (December 2016). "RNase H enables efficient repair of R-loop induced DNA damage". eLife. 5: e20533. doi:10.7554/eLife.20533. PMC 5215079. PMID 27938663.
- Lima WF, Murray HM, Damle SS, Hart CE, Hung G, De Hoyos CL, et al. (June 2016). "Viable RNaseH1 knockout mice show RNaseH1 is essential for R loop processing, mitochondrial and liver function". Nucleic Acids Research. 44 (11): 5299–312. doi:10.1093/nar/gkw350. PMC 4914116. PMID 27131367.
- Arudchandran A, Cerritelli S, Narimatsu S, Itaya M, Shin DY, Shimada Y, Crouch RJ (October 2000). "The absence of ribonuclease H1 or H2 alters the sensitivity of Saccharomyces cerevisiae to hydroxyurea, caffeine and ethyl methanesulphonate: implications for roles of RNases H in DNA replication and repair". Genes to Cells. 5 (10): 789–802. doi:10.1046/j.1365-2443.2000.00373.x. PMID 11029655.
- Cerritelli SM, Frolova EG, Feng C, Grinberg A, Love PE, Crouch RJ (March 2003). "Failure to produce mitochondrial DNA results in embryonic lethality in Rnaseh1 null mice". Molecular Cell. 11 (3): 807–15. doi:10.1016/s1097-2765(03)00088-1. PMID 12667461.
- Reyes A, Melchionda L, Nasca A, Carrara F, Lamantea E, Zanolini A, et al. (July 2015). "RNASEH1 Mutations Impair mtDNA Replication and Cause Adult-Onset Mitochondrial Encephalomyopathy". American Journal of Human Genetics. 97 (1): 186–93. doi:10.1016/j.ajhg.2015.05.013. PMC 4572567. PMID 26094573.
- Hollis T, Shaban NM (2011-01-01). Nicholson AW (ed.). Ribonucleases. Nucleic Acids and Molecular Biology. Springer Berlin Heidelberg. pp. 299–317. doi:10.1007/978-3-642-21078-5_12. ISBN 978-3-642-21077-8.
- Chon H, Vassilev A, DePamphilis ML, Zhao Y, Zhang J, Burgers PM, et al. (January 2009). "Contributions of the two accessory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex". Nucleic Acids Research. 37 (1): 96–110. doi:10.1093/nar/gkn913. PMC 2615623. PMID 19015152.
- Reijns MA, Jackson AP (August 2014). "Ribonuclease H2 in health and disease". Biochemical Society Transactions. 42 (4): 717–25. doi:10.1042/BST20140079. PMID 25109948.
- Chon H, Vassilev A, DePamphilis ML, Zhao Y, Zhang J, Burgers PM, et al. (January 2009). "Contributions of the two accessory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex". Nucleic Acids Research. 37 (1): 96–110. doi:10.1093/nar/gkn913. PMC 2615623. PMID 19015152.
- Wahba L, Amon JD, Koshland D, Vuica-Ross M (December 2011). "RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability". Molecular Cell. 44 (6): 978–88. doi:10.1016/j.molcel.2011.10.017. PMC 3271842. PMID 22195970.
- Kim N, Huang SN, Williams JS, Li YC, Clark AB, Cho JE, et al. (June 2011). "Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I". Science. 332 (6037): 1561–4. Bibcode:2011Sci...332.1561K. doi:10.1126/science.1205016. PMC 3380281. PMID 21700875.
- Ohtani N, Haruki M, Morikawa M, Crouch RJ, Itaya M, Kanaya S (January 1999). "Identification of the genes encoding Mn2+-dependent RNase HII and Mg2+-dependent RNase HIII from Bacillus subtilis: classification of RNases H into three families". Biochemistry. 38 (2): 605–18. doi:10.1021/bi982207z. PMID 9888800.
- Kochiwa H, Tomita M, Kanai A (July 2007). "Evolution of ribonuclease H genes in prokaryotes to avoid inheritance of redundant genes". BMC Evolutionary Biology. 7: 128. doi:10.1186/1471-2148-7-128. PMC 1950709. PMID 17663799.
- Rosta E, Yang W, Hummer G (February 2014). "Calcium inhibition of ribonuclease H1 two-metal ion catalysis". Journal of the American Chemical Society. 136 (8): 3137–44. doi:10.1021/ja411408x. PMC 3985467. PMID 24499076.
- Klumpp K, Hang JQ, Rajendran S, Yang Y, Derosier A, Wong Kai In P, et al. (December 2003). "Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors". Nucleic Acids Research. 31 (23): 6852–9. doi:10.1093/nar/gkg881. PMC 290251. PMID 14627818.
- Yang W, Lee JY, Nowotny M (April 2006). "Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity". Molecular Cell. 22 (1): 5–13. doi:10.1016/j.molcel.2006.03.013. PMID 16600865.
- Oda Y, Yamazaki T, Nagayama K, Kanaya S, Kuroda Y, Nakamura H (May 1994). "Individual ionization constants of all the carboxyl groups in ribonuclease HI from Escherichia coli determined by NMR". Biochemistry. 33 (17): 5275–84. doi:10.1021/bi00183a034. PMID 7909691.
- De Vivo M, Dal Peraro M, Klein ML (August 2008). "Phosphodiester cleavage in ribonuclease H occurs via an associative two-metal-aided catalytic mechanism". Journal of the American Chemical Society. 130 (33): 10955–62. doi:10.1021/ja8005786. PMC 2745632. PMID 18662000.
- Reus K, Mayer J, Sauter M, Scherer D, Müller-Lantzsch N, Meese E (March 2001). "Genomic organization of the human endogenous retrovirus HERV-K(HML-2.HOM) (ERVK6) on chromosome 7". Genomics. 72 (3): 314–20. doi:10.1006/geno.2000.6488. PMID 11401447.
- Ustyantsev K, Blinov A, Smyshlyaev G (14 March 2017). "Convergence of retrotransposons in oomycetes and plants". Mobile DNA. 8 (1): 4. doi:10.1186/s13100-017-0087-y. PMC 5348765. PMID 28293305.
- Ustyantsev K, Novikova O, Blinov A, Smyshlyaev G (May 2015). "Convergent evolution of ribonuclease h in LTR retrotransposons and retroviruses". Molecular Biology and Evolution. 32 (5): 1197–207. doi:10.1093/molbev/msv008. PMC 4408406. PMID 25605791.
- Malik HS (2005). "Ribonuclease H evolution in retrotransposable elements". Cytogenetic and Genome Research. 110 (1–4): 392–401. doi:10.1159/000084971. PMID 16093691.
- Figiel M, Chon H, Cerritelli SM, Cybulska M, Crouch RJ, Nowotny M (March 2011). "The structural and biochemical characterization of human RNase H2 complex reveals the molecular basis for substrate recognition and Aicardi-Goutières syndrome defects". The Journal of Biological Chemistry. 286 (12): 10540–50. doi:10.1074/jbc.M110.181974. PMC 3060507. PMID 21177858.
- Orcesi S, La Piana R, Fazzi E (2009). "Aicardi-Goutieres syndrome". British Medical Bulletin. 89: 183–201. doi:10.1093/bmb/ldn049. PMID 19129251.
- Crow YJ, Manel N (July 2015). "Aicardi-Goutières syndrome and the type I interferonopathies". Nature Reviews. Immunology. 15 (7): 429–40. doi:10.1038/nri3850. PMID 26052098.
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GM, Gornall HL, et al. (February 2015). "Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1". American Journal of Medical Genetics. Part A. 167A (2): 296–312. doi:10.1002/ajmg.a.36887. PMC 4382202. PMID 25604658.
- Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, et al. (October 2007). "Clinical and molecular phenotype of Aicardi-Goutieres syndrome". American Journal of Human Genetics. 81 (4): 713–25. doi:10.1086/521373. PMC 2227922. PMID 17846997.
- Sarafianos SG, Das K, Tantillo C, Clark AD, Ding J, Whitcomb JM, et al. (March 2001). "Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA". The EMBO Journal. 20 (6): 1449–61. doi:10.1093/emboj/20.6.1449. PMC 145536. PMID 11250910.
- Seeger C, Mason WS (May 2015). "Molecular biology of hepatitis B virus infection". Virology. 479-480: 672–86. doi:10.1016/j.virol.2015.02.031. PMC 4424072. PMID 25759099.
- Moelling K, Broecker F, Kerrigan JE (2014-01-01). "RNase H: specificity, mechanisms of action, and antiviral target". In Vicenzi E, Poli G (eds.). Human Retroviruses. Methods in Molecular Biology. 1087. Humana Press. pp. 71–84. doi:10.1007/978-1-62703-670-2_7. ISBN 978-1-62703-669-6. PMID 24158815.
- Mizuno M, Yasukawa K, Inouye K (February 2010). "Insight into the mechanism of the stabilization of moloney murine leukaemia virus reverse transcriptase by eliminating RNase H activity". Bioscience, Biotechnology, and Biochemistry. 74 (2): 440–2. doi:10.1271/bbb.90777. PMID 20139597. S2CID 28110533.
- Coté ML, Roth MJ (June 2008). "Murine leukemia virus reverse transcriptase: structural comparison with HIV-1 reverse transcriptase". Virus Research. 134 (1–2): 186–202. doi:10.1016/j.virusres.2008.01.001. PMC 2443788. PMID 18294720.
- Nowotny M, Figiel M (2013-01-01). LeGrice S, Gotte M (eds.). Human Immunodeficiency Virus Reverse Transcriptase. Springer New York. pp. 53–75. doi:10.1007/978-1-4614-7291-9_3. ISBN 978-1-4614-7290-2.
- Beilhartz GL, Götte M (April 2010). "HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors". Viruses. 2 (4): 900–26. doi:10.3390/v2040900. PMC 3185654. PMID 21994660.
- Klarmann GJ, Hawkins ME, Le Grice SF (2002). "Uncovering the complexities of retroviral ribonuclease H reveals its potential as a therapeutic target". AIDS Reviews. 4 (4): 183–94. PMID 12555693.
- Tramontano E, Di Santo R (2010). "HIV-1 RT-associated RNase H function inhibitors: Recent advances in drug development". Current Medicinal Chemistry. 17 (26): 2837–53. doi:10.2174/092986710792065045. PMID 20858167.
- Cao L, Song W, De Clercq E, Zhan P, Liu X (June 2014). "Recent progress in the research of small molecule HIV-1 RNase H inhibitors". Current Medicinal Chemistry. 21 (17): 1956–67. doi:10.2174/0929867321666140120121158. PMID 24438523.
- Ma BG, Chen L, Ji HF, Chen ZH, Yang FR, Wang L, et al. (February 2008). "Characters of very ancient proteins". Biochemical and Biophysical Research Communications. 366 (3): 607–11. doi:10.1016/j.bbrc.2007.12.014. PMID 18073136.
- Brindefalk B, Dessailly BH, Yeats C, Orengo C, Werner F, Poole AM (March 2013). "Evolutionary history of the TBP-domain superfamily". Nucleic Acids Research. 41 (5): 2832–45. doi:10.1093/nar/gkt045. PMC 3597702. PMID 23376926.
- Nichols NM, Yue D (2001-01-01). Ribonucleases. Current Protocols in Molecular Biology. Chapter 3. John Wiley & Sons, Inc. pp. Unit3.13. doi:10.1002/0471142727.mb0313s84. ISBN 978-0-471-14272-0. PMID 18972385.
- Loo JF, Wang SS, Peng F, He JA, He L, Guo YC, et al. (July 2015). "A non-PCR SPR platform using RNase H to detect MicroRNA 29a-3p from throat swabs of human subjects with influenza A virus H1N1 infection". The Analyst. 140 (13): 4566–75. Bibcode:2015Ana...140.4566L. doi:10.1039/C5AN00679A. PMID 26000345. S2CID 28974459.
- Goodrich TT, Lee HJ, Corn RM (April 2004). "Direct detection of genomic DNA by enzymatically amplified SPR imaging measurements of RNA microarrays". Journal of the American Chemical Society. 126 (13): 4086–7. CiteSeerX 10.1.1.475.1922. doi:10.1021/ja039823p. PMID 15053580.
- Dobosy JR, Rose SD, Beltz KR, Rupp SM, Powers KM, Behlke MA, Walder JA (August 2011). "RNase H-dependent PCR (rhPCR): improved specificity and single nucleotide polymorphism detection using blocked cleavable primers". BMC Biotechnology. 11: 80. doi:10.1186/1472-6750-11-80. PMC 3224242. PMID 21831278.
- Stein H, Hausen P (October 1969). "Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: effect on DNA-dependent RNA polymerase". Science. 166 (3903): 393–5. Bibcode:1969Sci...166..393S. doi:10.1126/science.166.3903.393. PMID 5812039.
- Hausen P, Stein H (June 1970). "Ribonuclease H. An enzyme degrading the RNA moiety of DNA-RNA hybrids". European Journal of Biochemistry. 14 (2): 278–83. doi:10.1111/j.1432-1033.1970.tb00287.x. PMID 5506170.
- Miller HI, Riggs AD, Gill GN (April 1973). "Ribonuclease H (hybrid) in Escherichia coli. Identification and characterization". The Journal of Biological Chemistry. 248 (7): 2621–4. PMID 4572736.
- Mölling K, Bolognesi DP, Bauer H, Büsen W, Plassmann HW, Hausen P (December 1971). "Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids". Nature. 234 (51): 240–3. doi:10.1038/newbio234240a0. PMID 4331605.
- Grandgenett DP, Gerard GF, Green M (December 1972). "Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses". Journal of Virology. 10 (6): 1136–42. doi:10.1128/jvi.10.6.1136-1142.1972. PMC 356594. PMID 4118867.
- Büsen W, Hausen P (March 1975). "Distinct ribonuclease H activities in calf thymus". European Journal of Biochemistry. 52 (1): 179–90. doi:10.1111/j.1432-1033.1975.tb03985.x. PMID 51794.
- Kanaya S, Crouch RJ (January 1983). "DNA sequence of the gene coding for Escherichia coli ribonuclease H". The Journal of Biological Chemistry. 258 (2): 1276–81. PMID 6296074.
- Itaya M (November 1990). "Isolation and characterization of a second RNase H (RNase HII) of Escherichia coli K-12 encoded by the rnhB gene". Proceedings of the National Academy of Sciences of the United States of America. 87 (21): 8587–91. Bibcode:1990PNAS...87.8587I. doi:10.1073/pnas.87.21.8587. PMC 55002. PMID 2172991.
- Ohtani N, Haruki M, Morikawa M, Crouch RJ, Itaya M, Kanaya S (January 1999). "Identification of the genes encoding Mn2+-dependent RNase HII and Mg2+-dependent RNase HIII from Bacillus subtilis: classification of RNases H into three families". Biochemistry. 38 (2): 605–18. doi:10.1021/bi982207z. PMID 9888800.
- Crouch RJ, Arudchandran A, Cerritelli SM (2001-01-01). "RNase H1 of Saccharomyces cerevisiae: methods and nomenclature". Methods in Enzymology. 341: 395–413. doi:10.1016/s0076-6879(01)41166-9. ISBN 978-0-12-182242-2. PMID 11582793.
- Frank P, Braunshofer-Reiter C, Wintersberger U, Grimm R, Büsen W (October 1998). "Cloning of the cDNA encoding the large subunit of human RNase HI, a homologue of the prokaryotic RNase HII". Proceedings of the National Academy of Sciences of the United States of America. 95 (22): 12872–7. Bibcode:1998PNAS...9512872F. doi:10.1073/pnas.95.22.12872. PMC 23637. PMID 9789007.
- Frank P, Braunshofer-Reiter C, Wintersberger U (January 1998). "Yeast RNase H(35) is the counterpart of the mammalian RNase HI, and is evolutionarily related to prokaryotic RNase HII". FEBS Letters. 421 (1): 23–6. doi:10.1016/s0014-5793(97)01528-7. PMID 9462832.
- Jeong HS, Backlund PS, Chen HC, Karavanov AA, Crouch RJ (2004-01-01). "RNase H2 of Saccharomyces cerevisiae is a complex of three proteins". Nucleic Acids Research. 32 (2): 407–14. doi:10.1093/nar/gkh209. PMC 373335. PMID 14734815.
External links
- GeneReviews/NCBI/NIH/UW entry on Aicardi-Goutières Syndrome
- RNase+H at the US National Library of Medicine Medical Subject Headings (MeSH)