Replication protein A
Replication protein A (RPA) is the major protein that binds to single-stranded DNA (ssDNA) in eukaryotic cells.[1][2] In vitro, RPA shows a much higher affinity for ssDNA than RNA or double-stranded DNA.[3]
| Replication protein A | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (heterotrimer) | |||||||||||||
 This is an image of human Replication protein A. From PDB: 1L1O Proteopedia protein A Replication protein A | |||||||||||||
| Function | damaged DNA binding, single-stranded DNA binding | ||||||||||||
| |||||||||||||
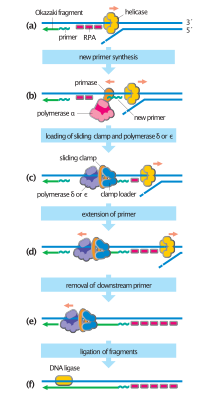
During DNA replication, RPA prevents single-stranded DNA (ssDNA) from winding back on itself or from forming secondary structures. This keeps DNA unwound for the polymerase to replicate it. RPA also binds to ssDNA during the initial phase of homologous recombination, an important process in DNA repair and prophase I of meiosis.
Hypersensitivity to DNA damaging agents can be caused by mutations in the RPA gene.[4] Like its role in DNA replication, this keeps ssDNA from binding to itself (self-complementizing) so that the resulting nucleoprotein filament can then be bound by Rad51 and its cofactors.[5]
RPA also binds to DNA during the nucleotide excision repair process. This binding stabilizes the repair complex during the repair process. A bacterial homolog is called single-strand binding protein (SSB).
Structure
RPA is a heterotrimer, composed of the subunits RPA1 (70kDa subunit), RPA2 (32kDa subunit) and RPA3 (14kDa subunit). The three RPA subunits contain four OB-folds (oligonucleotide/oligosaccharide binding) that bind RPA to single-stranded DNA.[2][3] RPA shares many features with the CST complex heterotrimer, although RPA has a more uniform 1:1:1 stoichiometry.[6]
See also
References
- Wold, MS (1997). "Replication protein A: heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism". Annual Review of Biochemistry. 66 (1): 61–92. doi:10.1146/annurev.biochem.66.1.61. PMID 9242902.
- Chen R, Wold MS (2014). "Replication protein A: single-stranded DNA's first responder: dynamic DNA-interactions allow replication protein A to direct single-strand DNA intermediates into different pathways for synthesis or repair". BioEssays. 36 (12): 1156–1161. doi:10.1002/bies.201400107. PMC 4629251. PMID 25171654.
- Flynn RL, Zou L (2010). "Oligonucleotide/oligosaccharide-binding fold proteins: a growing family of genome guardians". Critical Reviews in Biochemistry and Molecular Biology. 45 (4): 266–275. doi:10.3109/10409238.2010.488216. PMC 2906097. PMID 20515430.
- Zou, Yue; Liu, Yiyong; Wu, Xiaoming; Shell, Steven M. (2006-08-01). "Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses". Journal of Cellular Physiology. 208 (2): 267–273. doi:10.1002/jcp.20622. ISSN 0021-9541. PMC 3107514. PMID 16523492.
- Xuan, L; Wolf-Dietrich, H (2008). "Homologous recombination in DNA repair and DNA damage tolerance". Cell Research. 18 (99): 99–113. doi:10.1038/cr.2008.1. PMC 3087377. PMID 18166982.
- Lue NF, Zhou R, Chico L, Mao N, Steinberg-Neifach O, Ha T (2013). "The telomere capping complex CST has an unusual stoichiometry, makes multipartite interaction with G-Tails, and unfolds higher-order G-tail structures" (PDF). PLOS Genetics. 9 (1): e1003145. doi:10.1371/journal.pgen.1003145. PMC 3536697. PMID 23300477.