Dimefox
Dimefox was an organophosphate pesticide. In its pure form it is a colourless liquid with a fishy odour.[2] Dimefox was first produced in 1940 by the group of Gerhard Schrader in Germany. It was historically used as a pesticide, but has been deemed obsolete or discontinued for use by the World Health Organization. However, they do not guarantee that all commercial use of this compound ceased. But in most countries it is no longer registered for use as a pesticide.[3] It is considered an extremely hazardous substance as defined by the United States Emergency Planning and Community Right-to-Know Act.
 | |
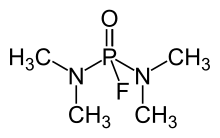 | |
| Names | |
|---|---|
| IUPAC name
N-[dimethylamino(fluoro)phosphoryl]-N-methylmethanamine | |
| Other names
tetramethylphosphorodiamidic fluoride bis(dimethylamino)fluorophosphine oxide bis(dimethylamido)phosphoryl fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.706 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H12FN2OP | |
| Molar mass | 154.125 g·mol−1 |
| Appearance | colourless liquid |
| Density | 1.11 g·mL–1 |
| 1000000 mg·L–1 | |
| Vapor pressure | 14663 mPa |
Henry's law constant (kH) |
2.28·10–8 atm·m3·mol–1[1] |
| Pharmacology | |
| inhalation and dermal contact | |
| Legal status |
|
| Hazards | |
| Main hazards | Corrosive (C), Highly Toxic (T+) |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R26/27/28 |
| S-phrases (outdated) | S1/2, S23, S28, S36/37, S38, S45 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
<2mg Oral |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8264
- "Archived copy". Archived from the original on 2011-07-22. Retrieved 2011-04-06.CS1 maint: archived copy as title (link)
- the WHO recommended classification of pesticides by hazard and guidelines to classification 2009, http://www.who.int/ipcs/publications/pesticides_hazard_2009.pdf
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.