Demeton
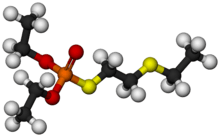
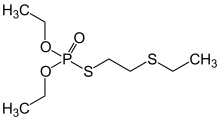
Demeton was a phosphorothioate insecticide with the chemical formula C8H19O3PS2. While it was previously used as an insecticide, it is now largely obsolete due to its relatively high toxicity to humans. The chemical structure of demeton is closely related to military nerve agents such as VX, and a derivative with one of the ethoxy groups replaced by methyl was investigated by both the US and Soviet chemical weapons programs under the names "V.sub.X" and "GD-7".[4][5]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
O,O-Diethyl S-2-(ethylsulfanyl)ethyl phosphorothioate | |
| Other names
Demeton thiol; Izosystox | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.366 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H19O3PS2 | |
| Molar mass | 258.3384 g/mol |
| Appearance | colorless to amber oily liquid[1] |
| Odor | sulfurous |
| Density | 1.146 g/cm3 |
| Boiling point | 128 °C (262 °F; 401 K) |
| 2.0 g/100 mL | |
| log P | 2.38[2] |
Refractive index (nD) |
1.5 |
| Hazards | |
| Flash point | 147.6 °C (297.7 °F; 420.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1.5 mg/kg (oral, rat) |
LDLo (lowest published) |
5 mg/kg (rabbit, oral) 7.85 mg/kg (mouse, oral) 1.7 mg/kg (rat, oral)[3] |
LCLo (lowest published) |
15 mg/m3 (rat, 4 hr) 15 mg/m3 (cat, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.1 mg/m3 [skin][1] |
REL (Recommended) |
TWA 0.1 mg/m3 [skin][1] |
IDLH (Immediate danger) |
10 mg/m3[1] |
| Related compounds | |
Related Organothiophosphate insecticides |
Demeton-S-methyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- Demeton-S-methyl
- Disulfoton, its phosphorodithioate equivalent
- V-sub x
References
- NIOSH Pocket Guide to Chemical Hazards. "#0177". National Institute for Occupational Safety and Health (NIOSH).
- "Demeton_msds".
- "Demeton". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Ledgard J. A laboratory history of chemical warfare agents (2nd, 2006). pp 230-233. ISBN 978-0-6151-3645-5
- Kulieva AM, Dalimov DN, Dorenskaya GM, et al. Biochemical investigation of cholinesterases and carboxylesterases from the cotton bollworm Heliothis armigera. Chem Nat Compd 1994 Jan; 30(1): 116-120. Kulieva, A. M; Dalimov, D. N; Dorenskaya, G. M; Charieva, O. V; Rozengart, V. I; Kugusheva, L. I; Moralev, S. N; Babaev, B. N; Abduvakhabov, A. A (1994). "Biochemical investigation of cholinesterases and carboxylesterases from the cotton bollworm Heliothis armigera". Chemistry of Natural Compounds. 30: 116–120. doi:10.1007/BF00638435.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.