Aluminium phosphide
Aluminium phosphide (aluminum phosphide) is a highly toxic inorganic compound with the chemical formula AlP used as a wide band gap semiconductor and a fumigant. This colorless solid is generally sold as a grey-green-yellow powder due to the presence of impurities arising from hydrolysis and oxidation.
 | |
| Names | |
|---|---|
| Other names
Aluminum phosphide Aluminium(III) phosphide Aluminium monophosphide Phostoxin Fumitoxin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.040.065 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1397 3048 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| AlP | |
| Molar mass | 57.9552 g/mol |
| Appearance | Yellow or gray crystals |
| Odor | garlic-like |
| Density | 2.85 g/cm3 |
| Melting point | 2,530 °C (4,590 °F; 2,800 K) |
| reacts | |
| Band gap | 2.5 eV (indirect)[1] |
Refractive index (nD) |
2.75 (IR), ~3 (Vis) [1] |
| Structure | |
| Zincblende | |
| T2d-F43m | |
a = 546.35 pm | |
| Tetrahedral | |
| Thermochemistry | |
Std molar entropy (S |
47.3 J/mol K |
Std enthalpy of formation (ΔfH⦵298) |
-164.4 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H260, H300, H311, H330, H400 |
| P223, P231+232, P260, P264, P270, P271, P273, P280, P284, P301+310, P302+352, P304+340, P310, P312, P320, P321, P322, P330, P335+334, P361, P363, P370+378, P391, P402+404, P403+233 | |
| NFPA 704 (fire diamond) | |
| Flash point | > 800 °C (1,470 °F; 1,070 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
11.5 mg/kg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
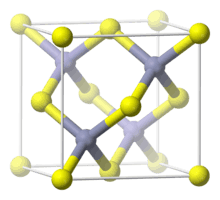
AlP crystals are dark grey to dark yellow in color and have a zincblende crystal structure[2] with a lattice constant of 5.4510 Å at 300 K.[3] They are thermodynamically stable up to 1,000 °C (1,830 °F).[4]
Aluminium phosphide reacts with water or acids to release phosphine:[5]
- AlP + 3 H2O → Al(OH)3 + PH3
- AlP + 3 H+ → Al3+ + PH3
Preparation
AlP is synthesized by combination of the elements:[4][6]
- 4Al + P4 → 4AlP
Caution must be taken to avoid exposing the AlP to any sources of moisture, as this generates toxic phosphine gas.
Uses
Pesticide
AlP is used as a rodenticide, insecticide, and fumigant for stored cereal grains. It is used to kill small verminous mammals such as moles and rodents. The tablets or pellets, known as "wheat pills", typically also contain other chemicals that evolve ammonia which helps to reduce the potential for spontaneous ignition or explosion of the phosphine gas.
AlP is used as both a fumigant and an oral pesticide. As a rodenticide, aluminium phosphide pellets are provided as a mixture with food for consumption by the rodents. The acid in the digestive system of the rodent reacts with the phosphide to generate the toxic phosphine gas. Other pesticides similar to aluminium phosphide are zinc phosphide and calcium phosphide. In this application, aluminium phosphide can be encountered under various brand names, e.g. Quickphos, Celphos, Fostox, Fumitoxin, Phostek, Phostoxin, Talunex, Fieldphos, and Weevil-Cide. It generates phosphine gas according to the following hydrolysis equation.[6]
- 2 AlP + 6 H2O → Al2O3∙3 H2O + 2 PH3
It is used as a fumigant when other pesticide applications are impractical and when structures and installations are being treated, such as in ships, aircraft, and grain silos. All of these structures can be effectively sealed or enclosed in a gastight membrane, thereby containing and concentrating the phosphine fumes. Fumigants are also applied directly to rodent burrows.[7]
Semiconductor applications
Industrially, AlP is a semiconductor material that is usually alloyed with other binary materials for applications in devices such as light-emitting diodes (e.g. aluminium gallium indium phosphide).[8]
Toxicology
Highly poisonous, aluminium phosphide has been used for suicide.[9] Fumigation has also caused unintentional deaths.[10][11][12] Known as "rice tablet" in Iran, for its use to preserve rice, there have been frequent incidents of accidental or intentional death. There is a campaign by the Iranian Forensic Medicine Organization to stop its use as a pesticide.[13][14]
Recycling of used aluminium phosphide containers caused the death of three family members in Alcalá de Guadaira, Spain. They had been keeping them in plastic sacks in their bathroom. The deaths occurred accidentally due to aluminum phosphide reacting with water or moisture, and becoming phosphine, leading to their death within hours.[15]
Aluminium phosphide poisoning is considered a wide-scale problem in the Indian subcontinent.[16][17]
References
- Berger, L. I. (1996). Semiconductor Materials. CRC Press. pp. 125. ISBN 0-8493-8912-7.
- Van Zeghbroeck; B. J. (1997). "Bravais Lattices; Zincblende Lattice". University of Colorado.
- "Lattice Constants". SiliconFarEast.com. 2004. Retrieved 3 January 2017.
- White, W. E.; Bushey, A. H.; Holtzclaw, H. F.; Hengeveld, F. W. (1953). Bailar, J. C. (ed.). "Aluminum Phosphide". Inorganic Syntheses. Inorganic Syntheses. 4: 23–25. doi:10.1002/9780470132357.ch7. ISBN 978-0-470-13235-7.
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- White, W. E.; Bushey, A. H. (1944). "Aluminum Phosphide – Preparation and Composition". Journal of the American Chemical Society. 66 (10): 1666. doi:10.1021/ja01238a018.
- Buckle, A. "Rodenticides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_211.
- Corbridge, D. E. C. (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- "Millionaire's death sparks poison scare". BBC News. 2002-10-10. Retrieved 2009-04-05.
- "Fumes kill two Danes in Jeddah". BBC News. 2009-02-24. Archived from the original on 25 February 2009. Retrieved 2009-02-25.
- "Family loses 2nd child in suspected pesticide poisoning". KSL-TV. 2010-02-09. Archived from the original on 11 February 2010.
- "4 children dead in Texas in pesticide spraying incident". CBS News. 2017-01-02.
- Shadnia, S.; Sasanian, G.; Allami, P.; Hosseini, A.; Ranjbar, A.; Amini-Shirazi, N.; Abdollahi, M. (2009). "A Retrospective 7-Years Study of Aluminum Phosphide Poisoning in Tehran: Opportunities for Prevention". Human & Experimental Toxicology. 28 (4): 209–213. doi:10.1177/0960327108097194. PMID 19734272.
- Mehrpour, O.; Singh, S. (2010). "Rice Tablet Poisoning: A Major Concern in Iranian Population". Human & Experimental Toxicology. 29 (8): 701–702. doi:10.1177/0960327109359643. PMID 20097728.
- "La familia de Alcalá de Guadaira murió tras inhalar plaguicida". La Vanguardia. Agencia EFE. 3 February 2014. Retrieved 3 February 2014.
- Siwach, SB; Gupta, A (1995). "The profile of acute poisonings in Harayana-Rohtak Study". The Journal of the Association of Physicians of India. 43 (11): 756–9. PMID 8773034.
- Singh, D; Jit, I; Tyagi, S (1999). "Changing trends in acute poisoning in Chandigarh zone: A 25-year autopsy experience from a tertiary care hospital in northern India". The American Journal of Forensic Medicine and Pathology. 20 (2): 203–10. doi:10.1097/00000433-199906000-00019. PMID 10414665.
