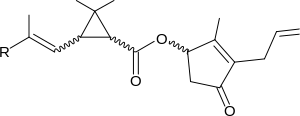
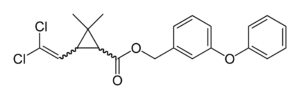
Pyrethroid
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (Chrysanthemum cinerariaefolium and C. coccineum). Pyrethroids are used as commercial and household insecticides.[1]
In household concentrations pyrethroids are generally harmless to humans.[1] However, pyrethroids are toxic to beneficial insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs.[2] Pyrethroids are toxic to aquatic organisms including fish.[3][4]
Mode of action
Pyrethroids are axonic excitotoxins, the toxic effects of which are mediated through preventing the closure of the voltage-gated sodium channels in the axonal membranes. The sodium channel is a membrane protein with a hydrophilic interior. This interior is shaped precisely to allow sodium ions to pass through the membrane, enter the axon, and propagate an action potential. When the toxin keeps the channels in their open state, the nerves cannot repolarize, leaving the axonal membrane permanently depolarized, thereby paralyzing the organism.[5] Pyrethroids can be combined with the synergist piperonyl butoxide, a known inhibitor of key microsomal cytochrome P450 enzymes from metabolizing the pyrethroid, which increases its efficacy (lethality).[6]
Chemistry and classification

Pyrethroids are mostly defined as pyrethroids based on their biological action, as they do not have common chemical structures. They often contain some 2,2-dimethylcyclopropanecarboxylic acid derivative, like chrysanthemic acid, which is esterified with a large alcohol. Cyclopropyl does not occur in all pyrethroids. Fenvalerate, which was developed in 1972, is one such example and was the first pyrethroid without the cyclopropyl group. Fenvalerate also has an α-cyano group. Pyrethroids which lack this α-cyano group are often classified as type I pyrethroids and those with it are called type II pyrethroids. Some pyrethroids, like etofenprox, lack the ester bond found from most pyrethroids and have an ether bond in its place. Silafluofen is also classified as a pyrethroid and it has silicon atom in the place of an ester. Pyrethroids often have chiral centers and only certain stereoisomers work efficiently as insecticides.[7]
Examples
- Allethrin, the first pyrethroid synthesized
- Bifenthrin, active ingredient of Talstar, Capture, Ortho Home Defense Max, and Bifenthrine
- Cyfluthrin, an active ingredient in Baygon, Temprid, Fumakilla Vape Aerosol, and many more, dichlorovinyl derivative of pyrethrin
- Cypermethrin, including the resolved isomer alpha-cypermethrin, dichlorovinyl derivative of pyrethrin
- Cyphenothrin, active ingredient of K2000 Insect spray sold in Israel and the Palestinian territories
- Deltamethrin, dibromovinyl derivative of pyrethrin
- Esfenvalerate
- Etofenprox
- Fenpropathrin
- Fenvalerate
- Flucythrinate
- Flumethrin
- Imiprothrin
- lambda-Cyhalothrin
- Metofluthrin
- Permethrin, dichlorovinyl derivative of pyrethrin and most widely used pyrethroid.
- Resmethrin, active ingredient of Scourge
- Silafluofen
- Sumithrin, active ingredient of Anvil
- tau-Fluvalinate
- Tefluthrin
- Tetramethrin
- Tralomethrin
- Transfluthrin, an active ingredient in Baygon
Environmental effects
Pyrethroids are toxic to beneficial insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs.[2] They are toxic to aquatic organisms including fish.[3][4]
Biodegradation
Pyrethroids are usually broken apart by sunlight and the atmosphere in one or two days, however when associated with sediment they can persist for some time.[8]
Pyrethroids are unaffected by conventional secondary treatment systems at municipal wastewater treatment facilities. They appear in the effluent, usually at levels lethal to invertebrates.[9]
Safety
Humans
Pyrethroid absorption can happen via skin, inhalation or ingestion.[10] Pyrethroids often do not bind efficiently to mammalian sodium channels.[11] They also absorb poorly via skin and human liver is often able to metabolize them relatively efficiently. Pyrethroids are thus much less toxic to humans than to insects.[12]
It is not well established if chronic exposure to small amounts of pyrethroids is hazardous or not.[13] However, large doses can cause acute poisoning, which is rarely life threatening. Typical symptoms include facial paresthesia, itching, burning, dizziness, nausea, vomiting and more severe cases of muscle twitching. Severe poisoning is often caused by ingestion of pyrethroids and can result in a variety of symptoms like seizures, coma, bleeding or pulmonary edema.[10]
Other organisms
Pyrethroids are very toxic to cats, but not to dogs. Poisoning in cats can result in seizures, fever, ataxia and even death. Poisoning can occur if pyrethroid containing flea treatment products, which are intended for dogs, are used on cats. The livers of cats detoxify pyrethroids via glucuronidation more poorly than dogs, which is the cause of this difference.[14] Aside from cats, pyrethroids are typically not toxic to mammals or birds.[15] They are often toxic to fish, reptiles and amphibians.[16]
Resistance
Although bedbugs were almost eradicated in North America through the use of DDT and organophosphates, populations of bedbugs resistant to both have developed. The use of DDT for this purpose was banned, and its reintroduction would not offer a solution to the problem of bedbugs, due to resistance.[17] Pyrethroids became more commonly used against bedbugs, but resistant populations have now developed to them as well.[18][19][20][21] Diamondback moths are also resistant to pyrethroids.[22]
History
Pyrethroids were introduced by a team of Rothamsted Research scientists in the 1960s and 1970s following the elucidation of the structures of pyrethrin I and II by Hermann Staudinger and Leopold Ružička in the 1920s.[23] The pyrethroids represented a major advancement in the chemistry that would synthesize the analog of the natural version found in pyrethrum. Its insecticidal activity has relatively low mammalian toxicity and an unusually fast biodegradation. Their development coincided with the identification of problems with DDT use. Their work consisted firstly of identifying the most active components of pyrethrum, extracted from East African chrysanthemum flowers and long known to have insecticidal properties. Pyrethrum rapidly knocks down flying insects but has negligible persistence — which is good for the environment but gives poor efficacy when applied in the field. Pyrethroids are essentially chemically stabilized forms of natural pyrethrum and belong to IRAC MoA group 3 (they interfere with sodium transport in insect nerve cells).[24]
The first-generation pyrethroids, developed in the 1960s, include bioallethrin, tetramethrin, resmethrin, and bioresmethrin. They are more active than the natural pyrethrum but are unstable in sunlight. With the 91/414/EEC review,[25] many 1st-generation compounds have not been included on Annex 1, probably because the market is not big enough to warrant the costs of re-registration (rather than any special concerns about safety).
By 1974, the Rothamsted team had discovered a second generation of more persistent compounds notably: permethrin, cypermethrin and deltamethrin. They are substantially more resistant to degradation by light and air, thus making them suitable for use in agriculture, but they have significantly higher mammalian toxicities. Over the subsequent decades these derivatives were followed with other proprietary compounds such as fenvalerate, lambda-cyhalothrin and beta-cyfluthrin. Most patents have now expired, making these compounds cheap and therefore popular (although permethrin and fenvalerate have not been re-registered under the 91/414/EEC process).
References
- Metcalf, Robert L (2000). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_263.
- Zaveri, Mihir (February 4, 2010). "Study Links Pesticides to River Contamination". The Daily Californian. The Daily Californian. Retrieved 9 June 2012.
- Thatheyus, A.J; Gnana Selvam, A.Deborah (2013). "Synthetic Pyrethroids: Toxicity and Biodegradation". Applied Ecology and Environmental Sciences. 1 (3): 33–6. doi:10.12691/aees-1-3-2.
- Pyrethroids fact sheet from the Illinois Department of Public Health.
- Soderlund, David M; Clark, John M; Sheets, Larry P; Mullin, Linda S; Piccirillo, Vincent J; Sargent, Dana; Stevens, James T; Weiner, Myra L (2002). "Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment". Toxicology. 171 (1): 3–59. doi:10.1016/s0300-483x(01)00569-8. PMID 11812616.
- Devine, G.J; Denholm, I (2009). "An unconventional use of piperonyl butoxide for managing the cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae)". Bulletin of Entomological Research. 88 (6): 601–10. doi:10.1017/S0007485300054262.
- Ujihara K (2019). "The history of extensive structural modifications of pyrethroids". Journal of Pesticide Science. 44 (4): 215–224. doi:10.1584/jpestics.D19-102. PMC 6861428. PMID 31777441.
- Luo, Yuzhou; Zhang, Minghua (2011). "Environmental Modeling and Exposure Assessment of Sediment-Associated Pyrethroids in an Agricultural Watershed". PLOS ONE. 6 (1): e15794. Bibcode:2011PLoSO...615794L. doi:10.1371/journal.pone.0015794. PMC 3016336. PMID 21246035.
- Weston, Donald P; Lydy, Michael J (2010). "Urban and Agricultural Sources of Pyrethroid Insecticides to the Sacramento-San Joaquin Delta of California". Environmental Science & Technology. 44 (5): 1833–40. Bibcode:2010EnST...44.1833W. doi:10.1021/es9035573. PMID 20121184.
- Bradberry, Sally M.; Cage, Sarah A.; Proudfoot, Alex T.; Vale, J. Allister (2005). "Poisoning due to pyrethroids". Toxicological Reviews. 24 (2): 93–106. doi:10.2165/00139709-200524020-00003. ISSN 1176-2551. PMID 16180929.
- Silver KS, et al. (2014). "Voltage-gated sodium channels as insecticide targets". Advances in Insect Physiology. 46: 389–433. doi:10.1016/B978-0-12-417010-0.00005-7. ISBN 9780124170100. PMC 6005695. PMID 29928068.
- Ray, David E.; Ray, Dr David; Forshaw, Philip J. (2000-01-01). "Pyrethroid Insecticides: Poisoning Syndromes, Synergies, and Therapy". Journal of Toxicology: Clinical Toxicology. 38 (2): 95–101. doi:10.1081/CLT-100100922. ISSN 0731-3810. PMID 10778904.
- Burns CJ, Pastoor TP (2018). "Pyrethroid epidemiology: a quality-based review". Critical Reviews in Toxicology. 48 (4): 297–311. doi:10.1080/10408444.2017.1423463. PMID 29389244.
- Boland LA, Angles JM (2010). "Feline permethrin toxicity: retrospective study of 42 cases". Journal of Feline Medicine and Surgery. 12 (2): 61–71. doi:10.1016/j.jfms.2009.09.018. ISSN 1532-2750. PMID 19897392.
- Gupta RC, et al. (2007). Veterinary toxicology: basic and clinical principles (1st ed.). Elsevier. pp. 676–677. doi:10.1016/B978-012370467-2/50153-X. ISBN 978-0-08-048160-9.
- Ortiz-Santaliestra ME, et al. (2018). "Validity of fish, birds and mammals as surrogates for amphibians and reptiles in pesticide toxicity assessment". Ecotoxicology. 27 (7): 819–833. doi:10.1007/s10646-018-1911-y. PMID 29492806.
- Craggs, Samantha (November 20, 2014). "DDT repeal would do nothing to combat bed bugs, experts say". CBC News. Retrieved 14 November 2016.
- Goddard, Jerome; Deshazo, R (2009). "Bed Bugs Cimex lectularius and Clinical Consequences of Their Bites". JAMA. 301 (13): 1358–66. doi:10.1001/jama.2009.405. PMID 19336711.
- Kolb, Adam; Needham, Glen R; Neyman, Kimberly M; High, Whitney A (2009). "Bedbugs". Dermatologic Therapy. 22 (4): 347–52. doi:10.1111/j.1529-8019.2009.01246.x. PMID 19580578.
- Voiland, Adam. "You May not be Alone" Archived 2011-11-07 at the Wayback Machine U.S. News & World Report 16 July 2007, Vol. 143, Issue 2, p53–54.
- Yoon, Kyong Sup; Kwon, Deok Ho; Strycharz, Joseph P; Hollingsworth, Craig S; Lee, Si Hyeock; Clark, J. Marshall (2008). "Biochemical and Molecular Analysis of Deltamethrin Resistance in the Common Bed Bug (Hemiptera: Cimicidae)". Journal of Medical Entomology. 45 (6): 1092–101. doi:10.1603/0022-2585(2008)45[1092:BAMAOD]2.0.CO;2. PMID 19058634.
- Leibee, Gary L.; Savage, Kenneth E. (1992). "Evaluation of Selected Insecticides for Control of Diamondback Moth and Cabbage Looper in Cabbage in Central Florida with Observations on Insecticide Resistance in the Diamondback Moth". The Florida Entomologist. 75 (4): 585. doi:10.2307/3496140. ISSN 0015-4040. JSTOR 3496140.
- Staudinger, H; Ruzicka, L (1924). "Insektentötende Stoffe I. Über Isolierung und Konstitution des wirksamen Teiles des dalmatinischen Insektenpulvers" [Insecticidal substances I. On isolation and constitution of the active part of the Dalmatian insect powder]. Helvetica Chimica Acta. 7 (1): 177–201. doi:10.1002/hlca.19240070124.
- Haddi, Khalid; Berger, Madeleine; Bielza, Pablo; Cifuentes, Dina; Field, Linda M; Gorman, Kevin; Rapisarda, Carmelo; Williamson, Martin S; Bass, Chris (2012). "Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta)". Insect Biochemistry and Molecular Biology. 42 (7): 506–13. doi:10.1016/j.ibmb.2012.03.008. PMID 22504519.
- "EUR-Lex - 31991L0414 - EN - EUR-Lex". europa.eu.